Co-Treatment of Winery and Domestic Wastewaters in Municipal Wastewater Treatment Plants: Analysis of Biodegradation Kinetics and Process Performance Impacts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Winery Wastewater
2.3. Mixed Liquor
2.4. Experimental Set-Up and Procedures
2.4.1. OUR Testing
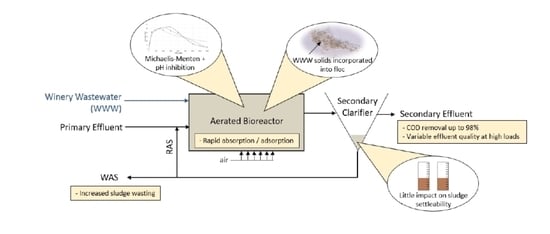
2.4.2. Aerobic Biological Treatment System
2.5. Analytical Methods
2.6. Determination of Kinetic Model Parameter Values
3. Results and Discussion
3.1. Winery Wastewater Quality and Respirometric Assessment
3.2. Aerobic Biodegradation Kinetics
3.2.1. Influence of pH on Biodegradation Rates
3.2.2. Kinetic Model Selection and Parameter Estimates
3.2.3. Influence of Aerobic Biomass Source on Biodegradation Rates
3.2.4. Evaluation of pH-Inhibition Model Accuracy and Fit
3.3. Aerobic Biological Co-Treatment of Winery and Domestic Wastewaters
3.3.1. Operating Conditions and Effluent Quality
3.3.2. Changes in Mixed Liquor Characteristics and Solids Yield
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APHA | American Public Health Association |
| BOD | biochemical oxygen demand |
| BOD5 | 5-day biochemical oxygen demand |
| CAS | conventional activated sludge |
| COD | chemical oxygen demand |
| Cpt | pseudo-toxic concentration |
| DO | dissolved oxygen |
| EA | extended aeration |
| MLSS | mixed liquor suspended solids |
| MLVSS | mixed liquor volatile suspended solids |
| MOE | Ministry of the Environment |
| SRT | solids retention time (d) |
| SVI | sludge volume index (mL/g) |
| TAN | total ammonia nitrogen |
| TKN | total Kjeldahl nitrogen |
| TOC | total organic carbon |
| TP | total phosphorus |
| TSS | total suspended solids |
| UCT | University of Cape Town |
| USEPA | United States Environmental Protection Agency |
| UVT | ultra-violet transmittance |
| VSS | volatile suspended solids |
| WAS | waste activated sludge |
| WEAO | Water Environment Association of Ontario |
| WWTP | wastewater treatment plant |
| WWW | winery wastewater |
| Symbols | |
| θ | Arrhenius temperature correction factor |
| μ | specific growth rate (h−1) |
| C | unitless variable |
| dissolved oxygen concentration (mg/L) | |
| Ks | half saturation constant (mg substrate/L) |
| OUR | oxygen uptake rate (mg O2/L·h) |
| pHref | pH at which inhibition effects first predicted |
| S | substrate concentration (mg/L) |
| SOUR | specific oxygen uptake rate (mg O2/g MLVSS·h) |
| t | time (h) |
| specific rate of substrate consumption (mg substrate/g VSS·h) | |
| X | biomass concentration (g VSS/L) |
| Y | biomass yield (g VSS/mg substrate or mg VSS/mg substrate) |
| Subscripts | |
| f | filtered |
| I | inhibited |
| max | maximum |
| o | initial value |
| T | temperature (°C) |
References
- Johnson, M.B.; Mehrvar, M. From Field to Bottle: Water Footprint Estimation in the Winery Industry. In Water Footprint; Muthu, S., Ed.; Springer Nature: Singapore, 2021; pp. 103–136. [Google Scholar] [CrossRef]
- Flores, L.; Garcia, J.; Pena, R.; Garfi, M. Constructed wetlands for winery wastewater treatment: A comparative Life Cycle Assessment. Sci. Total Environ. 2019, 659, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Masi, F.; Rochereau, J.; Troesch, S.; Ruiz, I.; Soto, M. Wineries wastewater treatment by constructed wetlands: A review. Water Sci. Technol. 2015, 71, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Mosse, K.P.M.; Patti, A.F.; Christen, E.W.; Cavagnaro, T.R. Review: Winery wastewater quality and treatment options in Australia. Aust. J. Grape Wine Res. 2011, 17, 111–122. [Google Scholar] [CrossRef]
- Bolzonella, D.; Papa, M.; Da Ros, C.; Muthukumar, L.A.; Rosso, D. Winery wastewater treatment: A critical overview of advanced biological processes. Crit. Rev. Biotechnol. 2019, 39, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Lofrano, G.; Meric, S. A comprehensive approach to winery wastewater treatment: A review of the state-of-the-art. Desalin. Water Treat. 2016, 57, 3011–3028. [Google Scholar] [CrossRef]
- Johnson, M.B.; Mehrvar, M. An assessment of the grey water footprint of winery wastewater in the Niagara Region of Ontario, Canada. J. Clean. Prod. 2019, 214, 623–632. [Google Scholar] [CrossRef]
- Johnson, M.B.; Mehrvar, M. Winery wastewater management and treatment in the Niagara Region of Ontario, Canada: A review and analysis of current regional practices and treatment performance. Can. J. Chem. Eng. 2020, 98, 5–24. [Google Scholar] [CrossRef]
- Bolzonella, D.; Zanette, M.; Battistoni, P.; Cecchi, F. Treatment of winery wastewater in a conventional municipal activated sludge process: Five years of experience. Water Sci. Technol. 2007, 56, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Prades, G.; Sadowski, A.-G. Activated sludge wastewater treatment plants optimisation to face pollution overloads during grape harvest periods. Water Sci. Technol. 2005, 51, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Vallier, K. “The Futre of Ontario’s Wine Industry,” The Niagara Independent, 8 March 2019. Available online: https://niagaraindependent.ca/the-future-of-ontarios-wine-industry/ (accessed on 24 January 2020).
- Metcalf and Eddy. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill: New York, NY, USA, 2014; ISBN 978-0-07-340118-8. [Google Scholar]
- Friedrich, M.; Takacs, I. A new interpretation of endogenous respiration profiles for the evaluation of the endogenous decay rate of heterotrophic biomass in activated sludge. Water Res. 2013, 47, 5639–5646. [Google Scholar] [CrossRef] [PubMed]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2012; ISBN 0875530133. [Google Scholar]
- Ko, J.H.; Choi, K.S.; Woo, H.J.; Lee, H.I.; Kim, C.W. Evaluation of pH inhibition effect on activated sludge by the pseudo toxic concentration (CPT) concept model. Water Sci. Technol. 2001, 43, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, R.D.; Sreekrishnan, T.R.; Campbell, P.G.C.; Blais, J.F. Kinetics of heavy metal bioleaching from sewage sludge—II. Mathematical model. Water Res. 1993, 27, 1653–1661. [Google Scholar] [CrossRef]
- UCT (University of Capetown). Theory, Design and Operation of Nutrient Removal Activated Sludge Process; Water Research Commission, WRC Report No. TT16/84; UCT: Pretoria, South Africa, 1984. [Google Scholar]
- USEPA. Process Design Manual for Nitrogen Control; Document ED 162 870; USEPA: Walnut Creek, CA, USA, 1975. [Google Scholar]
- Johnson, M.B.; Mehrvar, M. Characterising winery wastewater composition to optimise treatment and reuse. Aust. J. Grape Wine Res. 2020, 26, 410–416. [Google Scholar] [CrossRef]
- Conradie, A.; Sigge, G.O.; Cloete, T.E. Influence of winemaking practices on the characteristics of winery wastewater and water usage of wineries. S. Afr. J. Enol. Vitic. 2013, 35, 10–19. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Paredes, C.; Moral, R.; Moreno-Caselles, J.; Perez-Espinosa, A.; Perez-Murcia, M.D. Uses of winery and distillery effluents in agriculture: Characterisation of nutrient and hazardous components. Water Sci. Technol. 2005, 51, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Pirra, A.; Sousa, J.; Arroja, L.; Capela, I. Biodegradation kinetics of winery wastewater from port wine production. Chem. Biochem. Eng. Q. 2011, 25, 493–499. [Google Scholar]
- Reynolds, T.D.; Richards, P.A. Unit Operations and Processes in Environmental Engineering, 2nd ed.; Cengage Learning: Stamford, CT, USA, 1996; ISBN 978-0-534-94884-9. [Google Scholar]
- Eckenfelder, W.W.; Musterman, J.L. Activated Sludge Treatment of Industrial Wastewater; Technomic Publishing Company Inc.: Lancaster, PA, USA, 1995; ISBN 1-56676-302-9. [Google Scholar]
- Ministry of the Environment. Design Guidelines for Sewage Works. 2008. Available online: https://www.ontario.ca/document/design-guidelines-sewage-works-0 (accessed on 1 October 2018).
- Grainger, K.; Tattersall, H. Wine Production: Vine to Bottle; Blackwell Publishing Ltd.: Oxford, UK, 2005; ISBN 978-14051-1365-6. [Google Scholar]
- Water Environment Association of Ontario, Ministry of the Environment, Environment Canada. Optimization Guidance Manual for Sewage Works; Water Environment Association of Ontario: Mississauga, ON, Canada, 2010. [Google Scholar]
- Mehrvar, M.; Johnson, M.B. Method and System for Pre-Treating High Strength Wastewater. International Patent Application No. PCT/CA2022/050507 (WO 2022/204823), 4 April 2022. [Google Scholar]







| Co-Treatment | |||||
|---|---|---|---|---|---|
| Parameter | Units | Kinetic Trials | Trial 1 | Trial 2 | Trial 3 |
| BOD5 | mg/L | 43,600 | 140,000 | 58,900 | 42,000 |
| Filtered BOD5 | mg/L | 41,500 | - | 44,000 | 36,960 |
| COD | mg/L | 201,000 | 265,000 | 104,400 | 118,000 |
| Filtered COD | mg/L | 103,000 | 228,000 | 64,000 | 68,800 |
| TOC | mg/L | 25,000 | 39,100 | 35,600 | 26,400 |
| Filtered TOC | mg/L | 19,900 | - | 20,800 | 20,240 |
| TSS | mg/L | 45,200 | 37,200 | 12,080 | 24,800 |
| VSS | mg/L | 38,600 | 33,200 | 11,920 | 20,100 |
| TP | mg/L | 374 | 131 | 113 | 169 |
| Filtered TP | mg/L | 72.0 | 100 | 40.0 | 77.6 |
| TKN | mg/L | 300 | 473 | 644 | 900 |
| Filtered TKN | mg/L | 139 | 153 | 72.4 | 242 |
| pH | - | 3.68 | 3.60 | 4.86 | 3.90 |
| Identifier (for This Study) | Equation | Equation No. | Reference |
|---|---|---|---|
| UCT | (10) | [17] | |
| USEPA | (11) | [18] | |
| Cpt | (12) | [15] | |
| Tyagi | (13) | [16] |
| Facility | |||||
|---|---|---|---|---|---|
| Parameters | Units | A | B | C | D |
| Operating Conditions: | |||||
| Type of WWTP | - | CAS | CAS | CAS | EA |
| Co-treats WWW | - | Occasionally | Often | Never | Never |
| SRT | days | 5.9 | 10.7 | 21.4 | 11.4 |
| pHo | - | 8.39 | 7.92 | 8.04 | 7.96 |
| pHinhib,obs | - | 7.1–7.4 | 7.2–7.5 | n/a | 7.2–7.4 |
| Michaelis–Menten Parameters: | |||||
| mg COD/g MLVSS·h | 57.3 | 56.8 | 14.5 | 20.7 | |
| Ks | mg COD/L | 105 | 73.7 | 152 | 185 |
| MSE | (mg COD/g MLVSS·h)2 | 22.4 | 6.3 | 0.40 | 0.01 |
| UCT Parameters: | |||||
| pHref | - | 7.07 | 7.57 | 7.20 | 7.23 |
| C | - | 53.5 | 2.20 | 1.41 | 2.19 |
| MSE | (mg COD/g MLVSS·h)2 | 20.0 | 17.9 | 0.40 | 0.49 |
| USEPA Parameters: | |||||
| pHref | - | 7.31 | 8.14 | 7.25 | 7.42 |
| C | - | 1.10 | 0.296 | 0.277 | 0.462 |
| MSE | (mg COD/g MLVSS·h)2 | 18.1 | 22.6 | 0.29 | 0.61 |
| Cpt Parameters: | |||||
| pHref | - | 7.82 | 7.82 | 7.62 | 7.46 |
| C | - | 0.908 | 1.32 | 4.08 | 1.19 |
| MSE | (mg COD/g MLVSS·h)2 | 449 | 19.4 | 0.12 | 0.27 |
| Tyagi Parameters: | |||||
| pHref | - | 7.33 | 7.29 | 7.31 | 7.32 |
| C | - | −1.85 | −1.86 | −1.71 | −1.49 |
| MSE | (mg COD/g MLVSS·h)2 | 17.0 | 79.5 | 0.13 | 0.11 |
| Parameter | Units | Trial No. 1 | Trial No. 2 | Trial No. 3 |
|---|---|---|---|---|
| Total Duration | h | 6 | 6 | 72 |
| Initial MLSS Concentration | mg/L | 1260 | 1540 | 1470 |
| Initial MLVSS Concentration | mg/L | 1040 | 1180 | 1180 |
| Bioreactor Operation | ||||
| Temperature range | °C | 19.0 to 20.6 | 13.0 to 13.6 | 19.2 to 22.0 |
| Total operating volume | L | 14 | 12 | 16 |
| Volume of supernatant removed/feed added each cycle | L | 7 | 6 | 6 |
| Number of Feed Cycles | - | 1 | 1 | 5 1 |
| WWW added to Feed Mixture | ||||
| Volumetric Loadings Tested 2 | % v/v | 0.1; 0.5; 1.0 | 0.1; 0.5; 1.0; 2.0 | 0.1; 1.0 |
| Visual Characteristics 3 | - | Rose color, solids generally settleable, and no scum | White/green color, some solids settleable, and thick scum layer at surface | Rose color, solids generally settleable, and no scum |
| Primary Effluent Characteristics 4 | ||||
| BOD5 | mg/L | 74 | 27 | 65 |
| COD | mg/L | 226 | 113 | 263 |
| TOC | mg/L | 18.8 | 17.8 | 66 |
| TSS | mg/L | 100 | 33 | 80 |
| TKN | mg/L | 32.3 | 19.8 | 39.8 |
| TAN | mg/L | 16.2 | 14.2 | - |
| TP | mg/L | 3.9 | 1.4 | 4.8 |
| Parameter | Units | Control (0% v/v WWW) | 0.1% v/v WWW in Feed | 1.0% v/v WWW in Feed |
|---|---|---|---|---|
| Initial Conditions | ||||
| MLSS | mg/L | 1470 | 1470 | 1470 |
| MLVSS | mg/L | 1180 | 1180 | 1180 |
| Conditions at 60 h | ||||
| MLSS | mg/L | 1600 | 1720 | 2260 |
| MLVSS | mg/L | 1340 | 1440 | 1980 |
| Increase in MLSS at 60 h | mg/L | 130 | 250 | 790 |
| TSS Loading Fed to Bioreactor 1 | mg/L | 172 | 225 | 591 |
| Observed Yield | g VSS/g BOD5 | 1.15 | 1.67 | 3.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, M.B.; Mehrvar, M. Co-Treatment of Winery and Domestic Wastewaters in Municipal Wastewater Treatment Plants: Analysis of Biodegradation Kinetics and Process Performance Impacts. Sustainability 2023, 15, 6741. https://doi.org/10.3390/su15086741
Johnson MB, Mehrvar M. Co-Treatment of Winery and Domestic Wastewaters in Municipal Wastewater Treatment Plants: Analysis of Biodegradation Kinetics and Process Performance Impacts. Sustainability. 2023; 15(8):6741. https://doi.org/10.3390/su15086741
Chicago/Turabian StyleJohnson, Melody Blythe, and Mehrab Mehrvar. 2023. "Co-Treatment of Winery and Domestic Wastewaters in Municipal Wastewater Treatment Plants: Analysis of Biodegradation Kinetics and Process Performance Impacts" Sustainability 15, no. 8: 6741. https://doi.org/10.3390/su15086741
APA StyleJohnson, M. B., & Mehrvar, M. (2023). Co-Treatment of Winery and Domestic Wastewaters in Municipal Wastewater Treatment Plants: Analysis of Biodegradation Kinetics and Process Performance Impacts. Sustainability, 15(8), 6741. https://doi.org/10.3390/su15086741