Restoring Native Forest Understory: The Influence of Ferns and Light in a Hawaiian Experiment
Abstract
:1. Introduction

2. Methods
2.1. Study Site

2.2. Outplanting and Direct Seeding Experiments
2.2.1. Choice of Facilitator Species
2.2.2. Outplanting and Direct Seeding Species
| Hawaiian name | Latin name | Family | Habit | Seed size | Seed recalcitrant / orthodox? |
|---|---|---|---|---|---|
| hō'awa | Pittosporum hawaiianse | Pittosporaceae | Understory tree | Medium (1 mm < x < 8 mm) | orthodox |
| kōlea | Myrsine lessertiana | Myrsinaceae | Understory tree | Medium (1 mm < x < 8 mm) | recalcitrant |
| maile | Alyxia stellata | Apocynaceae | Liana | Large (>8 mm) | orthodox |
| māmaki | Pipturus albidus | Urticaceae | Shrub / small tree | Small (<1 mm) | orthodox |
| naio | Myoporum sandwichense | Scrophulariaceae | Understory tree | Large (>8 mm) | orthodox |
| pilo | Coprosma Montana | Rubiaceae | Understory tree | Medium (1 mm < x < 8 mm) | orthodox |
2.2.3. Study Design
| Fern Condition | |||
|---|---|---|---|
| F – Fern | N - No Fern | ||
| Light Condition | O - Open (High Light; 0–20% canopy cover with an average of 15%) | OF Treatment (Open, Fern) | ON Treatment (Open, No fern) |
| T - Tree (Medium Light; 20–80% canopy cover with an average of 50%) | TF Treatment (Tree, Fern) | TN Treatment (Tree, No fern) | |
| C - Canopy (Low Light; >80% canopy cover with an average of 88%) | CF Treatment (Canopy, Fern) | CN Treatment (Canopy, No fern) | |
2.2.4. Outplantings

2.2.5. Direct Seeding
2.2.6. Planting Technique
2.3. Monitoring and Ecological Data Collection
| Date of monitoring | Time elapsed since seedling outplanting | Time elapsed since seed planting |
|---|---|---|
| January 2010 | 6 months | n/a |
| July 2010 | 1 year | 2 months |
| Dec2010 | 1.5 years | 7 months |
| July 2011 | 2 years | 14 months |
| July 2012 | 3 years | 26 months |
2.4. Economic Data Collection
2.5. Statistical Analysis
| Question | Response variable(s) | Fixed Effects | Random Effects | Error Structure |
|---|---|---|---|---|
| Did seedling survivorship vary by treatment? | Survivorship at 3 years (Dead or Alive) | Treatment | Species, Plot | Binomial |
| Did seedling survivorship vary by species? | Survivorship at 3 years (Dead or Alive) | Species | Plot, Treatment | Binomial |
| Did seedling relative height vary by light level or fern? | Relative Height (Height at 3 yrs / Height at planting) | % Canopy, Fern presence/absence | Species, Plot | Gaussian |
| Did seedling relative height vary by species? | Relative Height (Height at 3 yrs / Height at planting) | Species | Plot, Treatment | Gaussian |
| Did seed germination rate vary by light level or fern presence? | Number of germinated seeds | % Canopy, Fern presence/absence* | Plot, Species | Poisson |
| Did seed germination rate vary by species? | Number of germinated seeds | Species | Plot, Treatment | Poisson |
| Fern | Light | Fern*Light | ||||||
|---|---|---|---|---|---|---|---|---|
| Height | Fern*light | 2074 | −0.951 | 0.3421 | −2.778 | 0.0057 | 1.102 | 0.271 |
| Fern + light | 2065 | 0.192 | 0.848 | −2.843 | 0.0046 | --- | --- | |
| Light | 2061 | --- | --- | −2.846 | 0.0046 | --- | --- | |
| Germination | Fern*light | 435 | −0.152 | 0.879 | 0.727 | 0.467 | 0.101 | 0.919 |
| Fern + light | 433 | −0.16 | 0.873 | 1.125 | 0.261 | --- | --- | |
| Light | 336 | --- | --- | 0.959 | 0.337 | --- | --- | |
| Survival | Fern*light | 688 | 0.498 | 0.619 | −4.804 | <0.0001 | 0.266 | 0.79 |
| Fern + light | 686 | 2.283 | 0.022 | −6.17 | <0.0001 | --- | --- | |
| Light | 689 | --- | --- | −6.23 | <0.0001 | --- | --- | |
3. Results
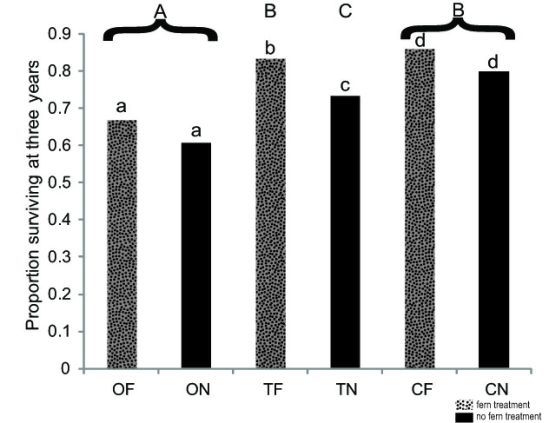
3.1 Seedling Survival

| Species | Outplanting Survivorship | Outplanting Relative Height (Average ± Std Dev.) | Seed Germination |
|---|---|---|---|
| M. sandwichense | 92%* | 3.8 (±1.9)a | 0.1% |
| A. stellata | 87%* | 1.7 (± 1.3)c | 15.0% |
| C. montana | 79%♦ | 3.7 (± 2.3)a | 2.1% |
| P. hawaiianse | 75%♦ | 3.0 (± 1.6)b | 0.8% |
| M. lessertiana | 73%♦ | 2.0 (± 1.0)c | 1.9% |
| P. albidus | 46%● | 3.0 (±1.3)b | 0.0% |
3.2. Seedling Relative Height
3.3. Seed Germination
3.4. Fern Size and Dieback
3.5. Costs of Various Planting Regimes
| Scenario Details | Outcomes | ||||
|---|---|---|---|---|---|
| Labor rate | Seed vs. Seedling | Light levels | Survivorship / Germination Rate | Cost per surviving seedling | |
| Scenario | |||||
| Outplanting, Volunteers, Shade | Low | Seedling | Canopy + Tree | 80.6% | $ 7.59 |
| Outplanting, Volunteers, All light | Low | Seedling | All light levels | 75.1% | $ 8.14 |
| Outplanting, Volunteers, Open and Tree | Low | Seedling | Open + Tree | 71.3% | $ 8.59 |
| Direct Seeding, no P. albidus, Volunteers, Shade | Low | Seed | Canopy + Tree | 2.9% | $ 9.25 |
| Outplanting, Volunteers, Open only | Low | Seedling | Open only | 64.2% | $ 9.53 |
| Direct Seeding, no P. albidus, Volunteers, All light | Low | Seed | All light levels | 2.8% | $ 9.56 |
| Direct Seeding, no P. albidus, Volunteers, Open and Tree | Low | Seed | Open + Tree | 2.7% | $ 9.72 |
| Direct Seeding, no P. albidus, Volunteers, Open only | Low | Seed | Open only | 2.6% | $ 10.25 |
| Outplanting, Paid Personnel, Shade | High | Seedling | Canopy + Tree | 80.6% | $ 12.63 |
| Outplanting, Paid Personnel, All light | High | Seedling | All light levels | 75.1% | $ 13.55 |
| Outplanting, Paid Personnel, Open and Tree | High | Seedling | Open + Tree | 71.3% | $ 14.29 |
| Outplanting, Paid Personnel, Open only | High | Seedling | Open only | 64.2% | $ 15.86 |
| Direct Seeding, no P. albidus, Paid Personnel, Shade | High | Seed | Canopy + Tree | 2.9% | $ 18.16 |
| Direct Seeding, no P. albidus, Paid Personnel, All light | High | Seed | All light levels | 2.8% | $ 18.77 |
| Direct Seeding, no P. albidus, Paid Personnel, Open and Tree | High | Seed | Open + Tree | 2.7% | $ 19.09 |
| Direct Seeding, no P. albidus, Paid Personnel, Open only | High | Seed | Open only | 2.6% | $ 20.11 |
4. Discussion
5. Conclusion
Supplementary Material
Acknowledgements
Conflict of Interest
References
- Enserink, M. Plan to quench the Everglades’ thirst. Science 1999, 285, 180. [Google Scholar] [CrossRef]
- U.S. Department of the Interior. The Department of the Interior’s Economic Contributions. Available online: http://www.doi.gov/ppa/upload/DOI-Econ-Report-6-21-2011.pdf (accessed on 28 February 2013).
- Nesshöver, C.; Aronson, J.; Blignaut, J. Chapter 9: Investing in Ecological Infrastructure. In TEEB—The Economics of Ecosystems and Biodiversity for National and International Policy Makers; Brink, P.T., Ed.; Routledge: New York, NY, USA, 2011. [Google Scholar]
- Liu, J.; Li, S.; Ouyang, Z.; Tam, C.; Chen, X. Ecological and socioeconomic effects of China’s policies for ecosystem services. Proc. Natl. Acad. Sci. USA 2008, 105, 9477–9482. [Google Scholar]
- Yin, R.; Yin, G.; Li, L. Assessing China’s Ecological Restoration Programs: What’s Been Done and What Remains to Be Done? Environ. Manage. 2010, 45, 442–453. [Google Scholar] [CrossRef]
- Hilderbrand, R.H.; Watts, A.C.; Randle, A.M. The myths of restoration ecology. Ecol. Soc. 2005, 10, 19–30. [Google Scholar]
- Roberts, L.; Stone, R.; Sugden, A. The rise of restoration ecology. Science 2009, 325, 555. [Google Scholar] [CrossRef]
- Goldman-Benner, R.L.; Benitez, S.; Boucher, T.; Calvache, A.; Daily, G.; Kareiva, P.; Kroeger, T.; Ramos, A. Water funds and payments for ecosystem services: practice learns from theory and theory can learn from practice. Oryx 2012, 46, 55. [Google Scholar] [CrossRef]
- Alexander, S.; Nelson, C.R.; Aronson, J.; Lamb, D.; Cliquet, A.; Erwin, K.L.; Finlayson, C.M.; De Groot, R.S.; Harris, J.A.; Higgs, E.S. Opportunities and challenges for ecological restoration within REDD+. Restor. Ecol. 2011, 19, 683–689. [Google Scholar] [CrossRef]
- Aide, T.M.; Zimmerman, J.K.; Pascarella, J.B.; Rivera, L.; Marcano-Vega, H. Forest Regeneration in a Chronosequence of Tropical Abandoned Pastures: Implications for Restoration Ecology. Restor. Ecol. 2000, 8, 328–338. [Google Scholar] [CrossRef]
- Craven, D.; Hall, J.; Verjans, J.M. Impacts of Herbicide Application and Mechanical Cleanings on Growth and Mortality of Two Timber Species in Saccharum spontaneum Grasslands of the Panama Canal Watershed. Restor. Ecol. 2009, 17, 751–761. [Google Scholar] [CrossRef]
- Fleming, G.M.; Diffendorfer, J.E.; Zedler, P.H. The relative importance of disturbance and exotic-plant abundance in California coastal sage scrub. Ecol. Appl. 2009, 19, 2210–2227. [Google Scholar] [CrossRef]
- Duncan, R.S.; Chapman, C.A. Limitations of animal seed dispersal for enhancing forest succession on degraded lands. In Seed Dispersal and Frugivory: Ecology, Evolution and Conservation; Levey, D.J., Silva, W.R., Galetti, M., Eds.; CAB International: Oxford, UK, 2002; pp. 437–450. [Google Scholar]
- Mendoza, I.; Gomez-Aparicio, L.; Zamora, R.; Matias, L. Recruitment limitation of forest communities in a degraded Mediterranean landscape. J. Veg. Sci. 2009, 20, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Moran, C.; Catterall, C.P.; Kanowski, J. Reduced dispersal of native plant species as a consequence of the reduced abundance of frugivore species in fragmented rainforest. Biol. Conserv. 2009, 142, 541–552. [Google Scholar] [CrossRef]
- Holl, K.D. Factors limiting tropical rain forest regeneration in abandoned pasture: Seed rain, seed germination, microclimate, and soil. Biotropica 1999, 31, 229–242. [Google Scholar] [CrossRef]
- Carpenter, F.L.; Mayorga, S.P.; Quintero, E.G.; Schroeder, M. Land-use and erosion of a Costa Rican Ultisol affect soil chemistry, mycorrhizal fungi and early regeneration. Forest Ecol. Manag. 2001, 144, 1–17. [Google Scholar] [CrossRef]
- Horiuchi, B.; Jeffrey, J. Native plant propagation and habitat restoration at Hakalau Forest National Wildlie Refuge, Hawaii. In Proceedings : Forest and Conservation Nursery Associations (19992000and 2001), Conferenence in Kailua-Kona, HI, USA, 21-25 August 2000; 2002; p. 233. [Google Scholar]
- Brooks, S.; Cordell, S.; Perry, L. Broadcast seeding as a potential tool to reestablish native species in degraded dry forest ecosystems in Hawaii. Ecol. Restor. 2009, 27, 300–305. [Google Scholar] [CrossRef]
- Goldstein, J.H.; Pejchar, L.; Daily, G.C. Using return-on-investment to guide restoration: a case study from Hawaii. Conserv. Lett. 2008, 1, 236–243. [Google Scholar] [CrossRef]
- Prach, K.; Pyšek, P. Using spontaneous succession for restoration of human-disturbed habitats: Experience from Central Europe. Ecol. Eng. 2001, 17, 55–62. [Google Scholar] [CrossRef]
- Wali, M. Ecological succession and the rehabilitation of disturbed terrestrial ecosystems. Plant Soil 1999, 213, 195–220. [Google Scholar] [CrossRef]
- Cusack, D.; Montagnini, F. The role of native species plantations in recovery of understory woody diversity in degraded pasturelands of Costa Rica. Forest Ecol. Manag. 2004, 188, 1–15. [Google Scholar] [CrossRef]
- Parrotta, J.A.; Turnbull, J.W.; Jones, N. Introduction–—Catalyzing native forest regeneration on degraded tropical lands. Forest Ecol. Manag. 1997, 99, 1–7. [Google Scholar] [CrossRef]
- Falk, D.A.; Palmer, M.A.; Zedler, J.B. Foundations of Restoration Ecology; Island Press: Washington, DC, USA, 2006. [Google Scholar]
- Kobe, R.K.; Pacala, S.W.; Silander, J.A.; Canham, C.D. Juvenile Tree Survivorship as a Component of Shade Tolerance. Ecol. App. 1995, 5, 517–532. [Google Scholar] [CrossRef]
- Loik, M.E.; Holl, K.D. Photosynthetic responses to light for rainforest seedlings planted in abandoned pasture, Costa Rica. Restor. Ecol. 1999, 7, 382–391. [Google Scholar] [CrossRef]
- Dos Santos, U.M.; Goncalves, J.F.D.; Feldpausch, T.R. Growth, leaf nutrient concentration and photosynthetic nutrient use efficiency in tropical tree species planted in degraded areas in central Amazonia. Forest Ecol. Manag. 2006, 226, 299–309. [Google Scholar] [CrossRef]
- Kelly, J.; Jose, S.; Nichols, J.D.; Bristow, M. Growth and physiological response of six Australian rainforest tree species to a light gradient. Forest Ecol. Manag. 2009, 257, 287–293. [Google Scholar] [CrossRef]
- Duncan, R.S.; Chapman, C.A. Tree-shrub interactions during early secondary forest succession in Uganda. Restor. Ecol. 2003, 11, 198–207. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. On the formation of dense understory layers in forests worldwide: consequences and implications for forest dynamics, biodiversity, and succession. Can. J. Forest Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L. The role of plant interactions in the restoration of degraded ecosystems: a meta-analysis across life-forms and ecosystems. J. Ecol 2009, 97, 1202–1214. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. The role of nurse plants in the restoration of degraded environments. Front Ecol Enviro 2006, 4, 196–202. [Google Scholar] [CrossRef]
- Graves, M.W.; Addison, D.J. The Polynesian settlement of the Hawaiian Archipelago: Integrating models and methods in archaeological interpretation. World Archaeol. 1995, 26, 380–399. [Google Scholar] [CrossRef]
- Cuddihy, L.W.; Stone, C.P. Alteration of Native HAWAIIAN Vegetation: Effects of Humans, Their Activities and Introductions; Cooperative Natl. Park Resources Studies Unit, Univ. Hawaii: Honolulu, HI, USA, 1990. [Google Scholar]
- Smith, C.W. Impact of alien plants on Hawaii’s native biota. In Hawaii’s Terrestrial Ecosystems: Preservation and Management; Scott, J.M., Stone, C.P., Eds.; University of Hawaiʻi: Honolulu, Hawaiʻi, 1985; pp. 180–250. [Google Scholar]
- Loope, L.L.; Giambelluca, T.W. Vulnerability of island tropical montane cloud forests to climate change, with special reference to East Maui, Hawaii. Climatic Change 1998, 39, 503–517. [Google Scholar] [CrossRef]
- Holt, A. An alliance of biodiversity, agriculture, health, and business interests for improved alien species management in Hawaii. In Invasive Species and Biodiversity Management; Sandlund, O.T., Schei, P.J., Viken, A.S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 65–75. [Google Scholar]
- George, L.O.; Bazzaz, F. The fern understory as an ecological filter: emergence and establishment of canopy-tree seedlings. Ecology 1999, 80, 833–845. [Google Scholar] [CrossRef]
- Ashton, M.S.; Gunatilleke, C.; Singhakumara, B.; Gunatilleke, I. Restoration pathways for rain forest in southwest Sri Lanka: a review of concepts and models. Forest Ecol. Manag. 2001, 154, 409–430. [Google Scholar] [CrossRef]
- Slocum, M.G.; Aide, T.M.; Zimmerman, J.K.; Navarro, L. Natural regeneration of subtropical montane forest after clearing fern thickets in the Dominican Republic. J. Tropl. Ecol. 2004, 20, 483–486. [Google Scholar]
- Giambelluca, T.W.; Chen, Q.; Frazier, A.G.; Price, J.P.; Chen, Y.-L.; Chu, P.-S.; Eischeid, J.K.; Delparte, D.M. Rainfall Atlas of Hawaiʻi. Bull. Amer. Meteor. Soc. 2012. [Google Scholar] [CrossRef]
- Wagner, W.L.; Herbst, D.R.; Sohmer, S.H. Manual of the Flowering Plants of Hawai'i: Revised Edition; Bishop Museum Press: Honolulu, HI, USA, 1999. [Google Scholar]
- USFWS, Revised Recovery Plan for the 'Alala (Corvus hawaiiensis). U.S. Fish & Wildlife Service: Portland, OR, USA, 2009.
- Sakai, H.F.; Carpenter, J.R. The Variety and Nutritional Value of Foods Consumed By Hawaiian Crow Nestlings, An Endangered Species. Condor 1990, 92, 220–228. [Google Scholar] [CrossRef]
- Culliney, S.M. Seed Dispersal by the Critically Endangered Alala (Corvus Hawaiiensis). Colorado State University: Fort Collins, CO, USA, 2011. [Google Scholar]
- Turnbull, L.A.; Rees, M.; Crawley, M.J. Seed mass and the competition/colonization trade-off: a sowing experiment. J. Ecol. 1999, 87, 899–912. [Google Scholar] [CrossRef]
- Woods, K.; Elliott, S. Direct seeding for forest restoration on abandoned agricultural land in Northern Thailand. J. Trop. For. Sci. 2004, 16, 2. [Google Scholar]
- Hau, H.C. Tree seed predation on degraded hillsides in Hong Kong. Forest Ecol. Manag. 1997, 99, 215–221. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. GLMM and GAMM. In Mixed Effects Models and Extensions in Ecology with R, 1st ed; Springer Science+Business Media, LLC: New York, NY, USA, 2009. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S.S. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Ostertag, R.; Cordell, S.; Michaud, J.; Cole, T.C.; Schulten, J.R.; Publico, K.M.; Enoka, J.H. Ecosystem and Restoration Consequences of Invasive Woody Species Removal in Hawaiian Lowland Wet Forest. Ecosystems 2009, 12, 503–515. [Google Scholar]
- Cordell, S.; McClellan, M.; Carter, Y.Y.; Hadwan, L.J. Towards restoration of Hawaiian tropical dry forests: the Kaupulehu outplanting programme. Pacific Conservation Biology 2008, 14, 279–284. [Google Scholar]
- Wester, L. Weed Management and the Habitat Protection of Rare species—A Case Study of the Endemic Hawaiian Fern Marsilea villosa. Biol.Conserv. 1994, 68, 1–9. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Wirawan, N. The Kahana Valley Ahupua'a, a PABITRA study Site on O'ahu, Hawaiian islands. Pac. Sci. 2005, 59, 293–314. [Google Scholar] [CrossRef] [Green Version]
- Griscom, H.P.; Griscom, B.W.; Ashton, M.S. Forest Regeneration from Pasture in the Dry Tropics of Panama: Effects of Cattle, Exotic Grass, and Forested Riparia. Forest Ecol. Manag. 2009, 17, 117–126. [Google Scholar]
- Aide, T.M.; Cavelier, J. Barriers to Lowland Tropical Forest Restoration in the Sierra Nevada de Santa Marta, Colombia. Restor. Ecol. 1994, 2, 219–229. [Google Scholar] [CrossRef]
- Funk, J.L.; Vitousek, P.M. Resource-use efficiency and plant invasion in low-resource systems. Nature 2007, 446, 1079–1081. [Google Scholar]
- Cordell, S.; Ostertag, R.; Rowe, B.; Sweinhart, L.; Vasquez-Radonic, L.; Michaud, J.; Colleen Cole, T.; Schulten, J.R. Evaluating barriers to native seedling establishment in an invaded Hawaiian lowland wet forest. Biol. Conserv. 2009, 142, 2997–3004. [Google Scholar] [CrossRef]
- Scowcroft, P.G.; Jeffrey, J. Potential significance of frost, topographic relief, and Acacia koa stands to restoration of mesic Hawaiian forests on abandoned rangeland. Forest Ecol. Manag. 1999, 114, 447–458. [Google Scholar] [CrossRef]
- Tunison, J.T.; D’Antonio, C.M.; Loh, R.K. Fire and Invasive Plants in Hawaiʻi Volcanoes National Park. In Proceedings of the Invasive Species Workshop: the Role of Fire in the Control and Spread of Invasive Species (Fire Conference 2000: the First National Congress on Fire Ecology, Prevention, and Management); Galley, K.E.M., Wilson, T.P., Eds.; Tall Timbers Research Station: Tallahassee, FL, USA, 2001. [Google Scholar]
- Giardina, C.P.; Litton, C.M.; Thaxton, J.M.; Cordell, S.; Hadway, L.J.; Sandquist, D.R. Science driven restoration: A candle in a demon haunted world—Response to Cabin. Restor. Ecol. 2007, 15, 171–176. [Google Scholar] [CrossRef]
- Cramer, V.A.; Hobbs, R.J.; Standish, R.J. What’s new about old fields? Land abandonment and ecosystem assembly. Trends Ecol. Evol. 2008, 23, 104–112. [Google Scholar] [CrossRef]
- Brauman, K.A.; Freyberg, D.L.; Daily, G.C. Forest structure influences on rainfall partitioning and cloud interception: A comparison of native forest sites in Kona, Hawai’i. Agr. Forest Meteorol. 2010, 150, 265–275. [Google Scholar] [CrossRef]
- Ticktin, T. The ecological implications of harvesting non-timber forest products. J. of Appl Ecol 2004, 41, 11–21. [Google Scholar] [CrossRef]
- Holl, K.D.; Loik, M.E.; Lin, E.H.V.; Samuels, I.A. Tropical montane forest restoration in Costa Rica: overcoming barriers to dispersal and establishment. Restor. Ecol. 2001, 8, 339–349. [Google Scholar]
- Cabin, R.J.; Weller, S.G.; Lorence, D.H.; Cordell, S.; Hadway, L.J.; Montgomery, R.; Goo, D.; Urakami, A. Effects of light, alien grass, and native species additions on Hawaiian dry forest restoration. Ecol. Appl. 2002, 12, 1595–1610. [Google Scholar] [CrossRef]
- Carpenter, L.F.; Nichols, D.; Sandi, E. Early growth of native and exotic trees planted on degraded tropical pasture. Forest Ecol. Manage. 2004, 196, 367–378. [Google Scholar] [CrossRef]
- Callaway, R.M. Positive Interactions and Interdependence in Plant Communities; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.J.; Anthelme, F.; et al. Facilitation in plant communities: the past, the present, and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar]
- Maestre, F.T.; Callaway, R.M.; Valladares, F.; Lortie, C.J. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 2009, 97, 199–205. [Google Scholar] [CrossRef]
- Cavieres, L.A.; Badano, E.I.; Sierra‐Almeida, A.; Gómez‐González, S.; Molina‐Montenegro, M.A. Positive interactions between alpine plant species and the nurse cushion plant Laretia acaulis do not increase with elevation in the Andes of central Chile. New Phytol. 2005, 169, 59–69. [Google Scholar]
- Gómez-Aparicio, L.; Zamora, R.; Gómez, J.M.; Hódar, J.A.; Castro, J.; Baraza, E. Applying plant facilitation to forest restoration: a meta-analysis of the use of shrubs as nurse plants. Ecol. Appl. 2004, 14, 1128–1138. [Google Scholar]
- Zanini, L.; Ganade, G. Restoration of Araucaria forest: the role of perches, pioneer vegetation, and soil fertility. Restor. Ecol. 2005, 13, 507–514. [Google Scholar] [CrossRef]
- Vieira, I.C.G.; Uhl, C.; Nepstad, D. The role of the shrub Cordia multispicata Cham. as a “succession facilitator” in an abandoned pasture, Paragominas, Amazonia. Vegetatio 1994, 115, 91–99. [Google Scholar]
- Duncan, R.S.; Chapman, C.A. Tree–shrub interactions during early secondary forest succession in Uganda. Restor. Eco. 2003, 11, 198–207. [Google Scholar] [CrossRef]
- Holl, K.D. Effect of shrubs on tree seedling establishment in an abandoned tropical pasture. J. Ecol. 2002, 90, 179–187. [Google Scholar] [CrossRef]
- Greenwell, S. The Changing Picture in Hawaiian Range Management. J. Range Manage 1959, 12, 99–103. [Google Scholar] [CrossRef]
- McDaniel, S.; Ostertag, R. Strategic light manipulation as a restoration strategy to reduce alien grasses and encourage native regeneration in Hawaiian mesic forests. Appl. Veg. Sci. 2010, 13, 280–290. [Google Scholar]
- Esquivel, M.J.; Harvey, C.A.; Finegan, B.; Casanoves, F.; Skarpe, C. Effects of pasture management on the natural regeneration of neotropical trees. J. Appl. Ecol. 2008, 45, 371–380. [Google Scholar]
- Denslow, J.S.; Uowolo, A.L.; Hughes, R.F. Limitations to seedling establishment in a mesic Hawaiian forest. Oecologia 2006, 148, 118–128. [Google Scholar] [CrossRef]
- Bonilla-Moheno, M.; Holl, K.D. Direct Seeding to Restore Tropical Mature-Forest Species in Areas of Slash-and-Burn Agriculture. Restor. Ecol. 2010, 18, 438–445. [Google Scholar] [CrossRef]
- NOAA. Drought Grips Hawaii in 2010. ClimateWatch Magazine 2011.
- Cole, R.J.; Holl, K.D.; Keene, C.; Zahawi, R.A. Direct seeding of late-successional trees to restore tropical montane forest. Forest Ecol Manag 2011, 261, 1590–1597. [Google Scholar] [CrossRef]
- Silvertown, J.W. Seed size, life span, and germination date as coadapted features of plant life history. Am. Nat. 1981, 860–864. [Google Scholar]
- Dalling, J.W.; Hubbell, S.P. Seed size, growth rate and gap microsite conditions as determinants of recruitment success for pioneer species. J. Ecol. 2002, 90, 557–568. [Google Scholar] [CrossRef]
- Palmerlee, A.P.; Young, T.P. Direct seeding is more cost effective than container stock across ten woody species in California. Native Plants J. 2010, 11, 89–102. [Google Scholar] [CrossRef]
- Minnesota DNR, Direct seeding of Native Hardwood Trees: An Innovative Approach to Hardwood Regeneration; Minnesota Department of Natural Resources: Minnesota, MN, USA, 2012.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gould, R.K.; Mooney, H.; Nelson, L.; Shallenberger, R.; Daily, G.C. Restoring Native Forest Understory: The Influence of Ferns and Light in a Hawaiian Experiment. Sustainability 2013, 5, 1317-1339. https://doi.org/10.3390/su5031317
Gould RK, Mooney H, Nelson L, Shallenberger R, Daily GC. Restoring Native Forest Understory: The Influence of Ferns and Light in a Hawaiian Experiment. Sustainability. 2013; 5(3):1317-1339. https://doi.org/10.3390/su5031317
Chicago/Turabian StyleGould, Rachelle K., Harold Mooney, Laura Nelson, Robert Shallenberger, and Gretchen C. Daily. 2013. "Restoring Native Forest Understory: The Influence of Ferns and Light in a Hawaiian Experiment" Sustainability 5, no. 3: 1317-1339. https://doi.org/10.3390/su5031317
APA StyleGould, R. K., Mooney, H., Nelson, L., Shallenberger, R., & Daily, G. C. (2013). Restoring Native Forest Understory: The Influence of Ferns and Light in a Hawaiian Experiment. Sustainability, 5(3), 1317-1339. https://doi.org/10.3390/su5031317