Constraining Factors in Hungarian Carp Farming: An Econometric Perspective
Abstract
:1. Introduction
Case Study of Hungarian Aquaculture
2. Materials and Methods
2.1. Data
2.2. Identification of Output and Input Variables for the Production Function
2.3. Specification of Production Functions
3. Results
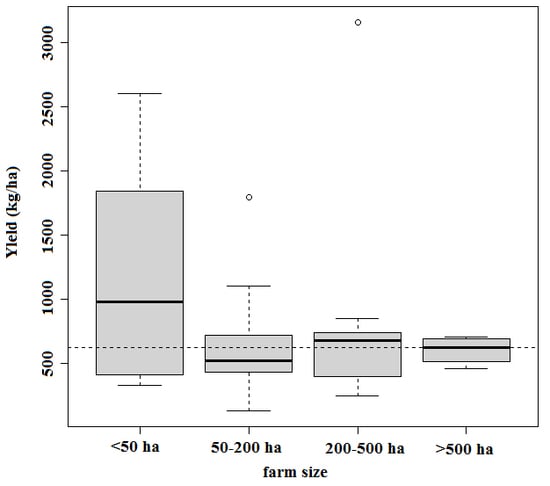
3.1. Descriptive Statistics
3.2. Econometric Model
4. Discussion
5. Conclusions
Acknowledgments

Author Contributions
Conflicts of Interest
References
- OECD (Organization for Economic Co-operation and Development); FAO (Food and Agriculture Organization of the United Nations). Agricultural Outlook 2017–2026; Organisation for Economic Co-operation and Development/Food and Agriculture Organisation of the United Nations: Paris, France, 2017. Available online: http://stats.oecd.org/Index.aspx?QueryId=76853 (accessed on 10 October 2017).
- OECD (Organization for Economic Co-operation and Development); FAO (Food and Agriculture Organization of the United Nations). Agricultural Outlook; Agriculture Statistics (Database); Organisation for Economic Co-operation and Development/Food and Agriculture Organisation of the United Nations: Rome, Italy, 2017. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Fishery and Aquaculture Statistics. Global Aquaculture Production 1950–2015; Food and Agriculture Organisation of the United Nations, Fisheries and Aquaculture Department: Rome, Italy, 2017. Available online: www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 10 October 2017).
- FAO (Food and Agriculture Organization of the United Nations). Regional Review on Aquaculture in the Asia-Pacific: Trends and Prospects—2010 FAO Fisheries and Aquaculture Circular; No. 1061/5. Food and Agriculture Organisation of the United Nations, Network of Aquaculture Centres in Asia-Pacific: Rome, Italy, 2011; p. 89. Available online: http://www.fao.org/docrep/014/i2311e/i2311e.pdf (accessed on 10 October 2017).
- Nandeesha, M.; Sentilkumar, V.; Antony Jesu Prabhu, P. Feed management of major carps in India, with special reference to practices adopted in Tamil Nadu. In On-Farm Feeding and Feed Management in Aquaculture; FAO Fisheries and Aquaculture Technical Paper No. 583; Hasan, M.R., New, M.B., Eds.; FAO: Rome, Italy, 2013; pp. 433–462. [Google Scholar]
- Wang, Q.; Cheng, L.; Liu, J.; Li, Z.; Xie, S.; De Silva, S.S. Freshwater aquaculture in PR China: Trends and prospects. Rev. Aquac. 2015, 7, 283–302. [Google Scholar] [CrossRef]
- Bostock, J.; Lane, A.; Hough, C.; Yamamoto, K. An assessment of the economic contribution of EU aquaculture production and the influence of policies for its sustainable development. Aquac. Int. 2016, 24, 699–733. [Google Scholar] [CrossRef]
- Adámek, Z.; Linhart, O.; Kratochvíl, M.; Flajšhans, M.; Randák, T.; Policar, T.; Kozák, P. Aquaculture in the Czech Republic in 2012: A prosperous and modern European sector based on a thousand-year history of pond culture. Aquac. Eur. 2012, 37, 5–14. [Google Scholar]
- Framian, N. Review of the EU Aquaculture Sector and Results of Costs and Earnings Survey, Part 1 of the Final Report on Definition of Data Collection Needs for Aquaculture. 2009. Available online: https://ec.europa.eu/fisheries/sites/fisheries/files/docs/body/aquadata_part1_en.pdf (accessed on 10 October 2017).
- Research Institute of Agricultural Economics. Report on the Fishery Sector; Research Institute for Agricultural Economics: Budapest, Hungary, 2016. Available online: https://www.aki.gov.hu/publikaciok/publikacio/a:116/Jelent%C3%A9s+a++hal%C3%A1szatr%C3%B3l (accessed on 10 October 2017).
- Mente, E.; Smaal, A. Introduction to the special issue on “European aquaculture development since 1993: The benefits of aquaculture to Europe and the perspectives of European aquaculture production”. Aquac. Int. 2016, 24, 693–698. [Google Scholar] [CrossRef]
- Edwards, P. Pilgrimage to traditional carp pond culture in Central Europe. Aquac. Asia 2007, 12, 28–34. [Google Scholar]
- Seiche, K.; Gerdeaux, D.; Gwiazda, R.; Levai, F.; Musil, P.; Nemenonck, O.; Strod, T.; Carss, D. Cormorant-Fisheries Conflicts at Carp Ponds in Europe and Israel—An INTERCAFE Overview; European Cooperation in Science and Technology: Wallingford, UK, 2012. Available online: http://www.intercafeproject.net/pdf/Carp_Ponds_Manual_FOR_WEB.pdf (accessed on 10 October 2017).
- Turkowski, K.; Lirski, A. The economics of carp farms in Poland. Acta Ichthyol. Piscat. 2010, 40, 137–144. [Google Scholar] [CrossRef]
- European Commission. Multiannual National Strategy Plan on Aquaculture of Hungary; European Commission: Brussels, Belgium, 2015. Available online: http://ec.europa.eu/fisheries/cfp/aquaculture/multiannual-national-plans_en (accessed on 10 October 2017).
- Asche, F. Farming the sea. Mar. Resour. Econ. 2008, 23, 527–547. [Google Scholar] [CrossRef]
- Asche, F.; Roll, K.H.; Sandvold, H.N.; Sørvig, A.; Zhang, D. Salmon aquaculture: Larger companies and increased production. Aquac. Econ. Manag. 2013, 17, 322–339. [Google Scholar] [CrossRef]
- Duffy, M. Economies of size in production agriculture. J. Hunger Environ. Nutr. 2009, 4, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Asche, F.; Nielsen, M. Restructuring European freshwater aquaculture from family-owned to large-scale firms—Lessons from Danish aquaculture. Aquac. Res. 2016, 47, 3852–3866. [Google Scholar] [CrossRef]
- Cornia, G.A. Farm size, land yields and the agricultural production function: An analysis for fifteen developing countries. World Dev. 1985, 13, 513–534. [Google Scholar] [CrossRef]
- Asche, F.; Roll, K.H.; Tveteras, R. Economic inefficiency and environmental impact: An application to aquaculture production. J. Environ. Econ. Manag. 2009, 58, 93–105. [Google Scholar] [CrossRef]
- Nilsen, O.B. Learning-by-doing or technological leapfrogging: Production frontiers and efficiency measurement in Norwegian salmon aquaculture. Aquac. Econ. Manag. 2010, 14, 97–119. [Google Scholar] [CrossRef]
- Salvanes, K.G. The structure of the Norwegian fish farming industry: An empirical analysis of economies of scale and substitution possibilities. Mar. Resour. Econ. 1989, 6, 349–373. [Google Scholar] [CrossRef]
- De Ionno, P.N.; Wines, G.L.; Jones, P.L.; Collins, R.O. A bioeconomic evaluation of a commercial scale recirculating finfish growout system—An Australian perspective. Aquaculture 2006, 259, 315–327. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Josupeit, H.; Cai, J.; Zhou, X. The role of crustacean fisheries and aquaculture in global food security: Past, present and future. J. Invertebr. Pathol. 2012, 110, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, T.; Salvanes, K.G. Gains from deregulation? An empirical test for efficiency gains in the Norwegian fish farming industry. J. Agric. Econ. 1995, 46, 113–126. [Google Scholar] [CrossRef]
- Váradi, L.; Lane, A.; Harache, Y.; Gyalog, G.; Békefi, E.; Lengyel, P. Regional Review on Status and Trends in Aquaculture Development in Europe, 2010; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2012; ISBN 9250068670. [Google Scholar]
- Engle, C.R. Aquaculture Economics and Financing: Management and Analysis; John Wiley & Sons: Ames, IA, USA, 2010; ISBN 978-0-8138-1301-1/2010. [Google Scholar]
- Hungarian Aquaculture and Fisheries Inter-Branch Organisation (MA-HAL). Annual Report 2014 of Hungarian Fish Farmers’ Association; Hungarian Aquaculture and Fisheries Inter-Branch Organisation (MA-HAL): Budapest, Hungary, 2015; p. 60. Available online: http://magyarhal.hu/eves-jelentes (accessed on 10 October 2017).
- Hungarian Central Statistical Office. Monthly Average Gross Wages of Physical Workers in the National Economy; Hungarian Central Statistical Office: Budapest, Hungary, 2017. Available online: http://www.ksh.hu/docs/hun/xstadat/xstadat_evkozi/e_qli008a.html (accessed on 10 October 2017).
- Lacewell, R.D.; Nichols, J.P.; Jambers, T.H. An analysis of pond raised catfish production in Texas. J. Agric. Appl. Econ. 1973, 5, 141–145. [Google Scholar] [CrossRef]
- Singh, K.; Dey, M.M.; Rabbani, A.G.; Sudhakaran, P.O.; Thapa, G. Technical Efficiency of Freshwater Aquaculture and its Determinants in Tripura, India. Agric. Econ. Res. Rev. 2009, 22, 185–195. [Google Scholar]
- Ali, H.; Murshed-e-Jahan, K.; Belton, B.; Dhar, G.C.; Rashid, H.O. Factors determining the productivity of mola carplet (Amblypharyngodon mola, Hamilton, 1822) in carp polyculture systems in Barisal district of Bangladesh. Aquaculture 2016, 465, 198–208. [Google Scholar] [CrossRef]
- Mohan Dey, M.; Javien Paraguas, F.; Srichantuk, N.; Xinhua, Y.; Bhatta, R.; Thi Chau Dung, L. Technical efficiency of freshwater pond polyculture production in selected Asian countries: Estimation and implication. Aquac. Econ. Manag. 2007, 9, 39–63. [Google Scholar] [CrossRef]
- Henningsen, A. Introduction to Econometric Production Analysis with R (Draft Version); Department of Food and Resource Economics, University of Copenhagen: Copenhagen, Denmark, 2014. [Google Scholar]
- Hendrickx, J. Perturb: Tools for Evaluating Collinearity, Software. Manual. 2015. Available online: https://cran.r-project.org/web/packages/perturb/perturb.pdf (accessed on 16 November 2017).
- Henningsen, A.; Toomet, T. miscTools: Miscellaneous Tools and Utilities. R Package Version 0.6-20. Manual. 2016. Available online: https://CRAN.R-project.org/package=miscTools (accessed on 16 November 2017).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Zeileis, A.; Hothorn, T. Diagnostic Checking in Regression Relationships. R News 2002, 2, 7–10. [Google Scholar]
- Kennedy, P. A Guide to Econometrics; Blackwell: Oxford, UK, 2008; ISBN 978-1-4051-8257-7. [Google Scholar]
- Gyalog, G.; Váradi, L.; Gál, D. Is intensification a viable way for pond culture in Central and Eastern Europe? AACL Bioflux 2011, 4, 584–589. [Google Scholar]
- Marković, Z.; Stanković, M.; Rašković, B.; Dulić, Z.; Živić, I.; Poleksić, V. Comparative analysis of using cereal grains and compound feed in semi-intensive common carp pond production. Aquac. Int. 2016, 24, 1699–1723. [Google Scholar] [CrossRef]
- Edwards, P. Aquaculture environment interactions: Past, present and likely future trends. Aquaculture 2015, 447, 2–14. [Google Scholar] [CrossRef]
- Tacon, A.G.; Metian, M. Feed matters: Satisfying the feed demand of aquaculture. Rev. Fish. Sci. Aquac. 2015, 23, 1–10. [Google Scholar] [CrossRef]
- Gorton, M.; Davidova, S. Farm productivity and efficiency in the CEE applicant countries: A synthesis of results. Agric. Econ. 2004, 30, 1–16. [Google Scholar] [CrossRef]
- Barrett, C.B. On price risk and the inverse farm size-productivity relationship. J. Dev. Econ. 1996, 51, 193–215. [Google Scholar] [CrossRef]


| Name of Variable | Calculated/Estimated Unit Value 1 | Calculation Method and Source of Information |
|---|---|---|
| Unit value of production | $2.3/kg | Average farm gate prices are reported by the [29]. Unit value of the overall output was calculated as the weighted average of farm gate values of different age classes of Common carp in 2014. |
| Unit cost of labour | $8877/Full-Time Equivalent | Unit cost of labour in pond-based carp farming was proxied by the average gross earnings of physical workers including social security taxes in the agricultural sector in 2014, as reported by the [30]. |
| Unit cost of feed | $0.174/kg | The average cost of feed (cereal grain) was estimated based on stock exchange prices prevailing in 2014. |
| Annual cost of capital investments in production infrastructure (ponds) | $424.5/hectare/year | The following assumptions were made based on expert estimations: (i) the average construction cost of ponds is $15,436/ha; (ii) there is a 50% investment subsidy rate available for pond construction; (iii) the Capital Recovery Factor is 5.5% (assuming a 5% interest rate and 50 years of useful life for ponds). To compute the annual cost of construction we multiplied these three items. |
| Variables | Units | Brief Description and Calculation Method of Variable |
|---|---|---|
| Gross production of Common carp (Y) | Tonnes | Harvested quantity in live weight |
| Raw Material (RM) | Tonnes (expressed in feed equivalent) | Combined variable to represent the two major operating inputs: feed and stocking material. Calculated as the quantity of feed (in t) plus 15 times the quantity of stocking material (in t). The unit value of fish seed is approximately 15 times that of feed. |
| Machinery (M) | Number of Large Machines | This variable is calculated as the sum of the following machinery items: weed and reed cutting mower boats; mechanical loaders and tractors. |
| Labour (L) | Full-Time-Equivalent (FTE) 1 | Combined variable aggregating full-time employees (Lf), part-time employees (Lp) and days actually worked per occasional worker (Lo). The FTE was calculated as Lf + Lp*1/2 + Lo*1/200 |
| Pond area (P) | Hectares | Pond area in use |
| Items | Survey Data (Mean ± SD) | Average 4 | |||
|---|---|---|---|---|---|
| <50 ha | 50–200 ha | 200–500 ha | >500 ha | ||
| Number of farms | 12 | 19 | 9 | 4 | |
| Gross yield (t/ha) | |||||
| Common carp (C.c) | 1.17 ± 0.77 | 0.64 ± 0.37 | 0.86 ± 0.83 | 0.61 ± 0.11 | 0.62 |
| All species (including C.c) | 1.30 ± 0.77 | 0.83 ± 0.37 | 1.11 ± 0.97 | 0.77 ± 0.09 | 0.78 |
| Net yield (t/ha) | |||||
| Common carp | 0.88 ± 0.66 | 0.38 ± 0.27 | 0.48 ± 0.43 | 0.35 ± 0.06 | 0.38 |
| All species (incl. C.c) | 0.97 ± 0.67 | 0.51 ± 0.29 | 0.66 ± 0.55 | 0.45 ± 0.06 | 0.49 |
| Stocking density (t/ha) | |||||
| Common carp | 0.30 ± 0.18 | 0.26 ± 0.14 | 0.38 ± 0.42 | 0.26 ± 0.06 | 0.24 |
| All species (incl. C.c) | 0.32 ± 0.18 | 0.33 ± 0.15 | 0.45 ± 0.45 | 0.32 ± 0.05 | 0.29 |
| Labour (FTE/ha 1) | 0.12 ± 0.11 | 0.04 ± 0.05 | 0.05 ± 0.03 | 0.05 ± 0.01 | 0.05 |
| Feed (t/ha) | 3.35 ± 2.18 | 1.72 ± 1.09 | 2.29 ± 1.55 | 1.67 ± 0.61 | 1.72 |
| Machinery (LMU/ha 2) | 0.06 ± 0.10 | 0.03 ± 0.03 | 0.02 ± 0.01 | 0.01 ± 0.00 | n.a. |
| Efficiency indicators | |||||
| Feed Conversion Rate | 5.07 ± 5.08 | 5.38 ± 2.96 | 5.65 ± 2.25 | 4.72 ± 1.45 | 4.48 |
| Ratio of harvested to stocked quantity of C.c. | 4.29 ± 1.95 | 2.60 ± 0.88 | 2.57 ± 0.77 | 2.38 ± 0.25 | 2.61 |
| Labour efficiency (t/FTE) 3 | 14.0 ± 12.3 | 16.65 ± 9.38 | 23.56 ± 21.2 | 13.1 ± 2.5 | n.a. |
| Total Carp Production per Farm (Ln Y) | Carp Yields per Hectare (Ln y) | ||||
|---|---|---|---|---|---|
| Parameters | Model 1 | Parameters | Model 2 | ||
| Y-intercept | −0.011 | (0.666) | Y-intercept | −0.094 | (0.652) |
| RM | 0.857 | (0.069) *** | rm | 0.859 | (0.069) *** |
| L | 0.201 | (0.065) ** | l | 0.219 | (0.060) ** |
| M | 0.033 | (0.022) | m | 0.034 | (0.023) |
| P | −0.117 | (0.089) | |||
| R2 | 0.956 | R2 | 0.808 | ||
| F-value | 231.7 *** | F-value | 61.48 *** | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyalog, G.; Oláh, J.; Békefi, E.; Lukácsik, M.; Popp, J. Constraining Factors in Hungarian Carp Farming: An Econometric Perspective. Sustainability 2017, 9, 2111. https://doi.org/10.3390/su9112111
Gyalog G, Oláh J, Békefi E, Lukácsik M, Popp J. Constraining Factors in Hungarian Carp Farming: An Econometric Perspective. Sustainability. 2017; 9(11):2111. https://doi.org/10.3390/su9112111
Chicago/Turabian StyleGyalog, Gergő, Judit Oláh, Emese Békefi, Mónika Lukácsik, and József Popp. 2017. "Constraining Factors in Hungarian Carp Farming: An Econometric Perspective" Sustainability 9, no. 11: 2111. https://doi.org/10.3390/su9112111
APA StyleGyalog, G., Oláh, J., Békefi, E., Lukácsik, M., & Popp, J. (2017). Constraining Factors in Hungarian Carp Farming: An Econometric Perspective. Sustainability, 9(11), 2111. https://doi.org/10.3390/su9112111