Spectral Responses of As and Pb Contamination in Tailings of a Hydrothermal Ore Deposit: A Case Study of Samgwang Mine, South Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.1.1. Geology and Ore Deposit
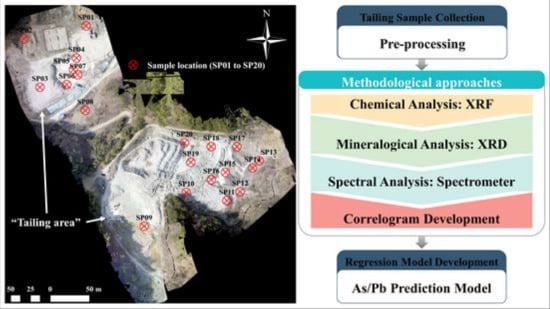
2.1.2. Mine Dumps
2.2. Sample Collection and Pre-Processing
2.3. Chemical and Mineralogical Analysis
2.4. Spectral Analysis
2.5. Model Development and Evaluation
3. Results
3.1. Heavy Metal Concentration and Mineral Composition
3.2. VNIR-SWIR Spectral Characteristics and Spectroscopy
Wavelength Selection
3.3. Prediction Models and Evaluations
3.3.1. Development of Stepwise Multiple Linear Regression Models
3.3.2. Model Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thornton, I. Soil contamination in urban areas. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1990, 82, 121–140. [Google Scholar] [CrossRef]
- Xian, X.; Shokohifard, G.I. Effect of pH on chemical forms and plant availability of cadmium, zinc, and lead in polluted soils. Water Air Soil Pollut. 1989, 45, 265–273. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Rashed, M.N. Monitoring of contaminated toxic and heavy metals, from mine tailings through age accumulation, in soil and some wild plants at southeast Egypt. J. Hazard. Mater. 2010, 178, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Kemper, T.; Sommer, S. Estimate of heavy metal contamination in soils after a mining accident using reflectance spectroscopy. Environ. Sci. Technol. 2002, 36, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Sources of heavy metals and metalloids in soils. In Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 2013; Volume 22, pp. 11–50. ISBN 978-94-007-4469-1. [Google Scholar]
- Wetzel, M.A.; Wahrendorf, D.-S.; Peter, C. Sediment pollution in the Elbe estuary and its potential toxicity at different trophic levels. Sci. Total Environ. 2013, 449, 199–207. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Proven Alternatives for Aboveground Treatment of Arsenic in Groundwater; EPA-542-S-02-002; Office of Solid Wastes and Emergency of United States Environmental Protection Agency: Seattle, WA, USA, 2002; pp. 1–38. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/arsenic_issue_paper.pdf (accessed on 22 August 2018).
- The Ministry of Environment of Korea. Survey on Soil Contamination in Waste Metal Mines (Chungnam Province); M045470; The Ministry of Environment of Korea: Seoul, Korea, 2005; pp. 5–14. Available online: http://webbook.me.go.kr/DLi-File/F004/000/141833.pdf (accessed on 22 August 2018).
- Rathod, P.H.; Rossiter, D.G.; Noomen, M.F.; Van der Meer, F.D. Proximal spectral sensing to monitor phytoremediation of metal-contaminated soils. Int. J. Phytoremediat. 2013, 15, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Chen, Y.; Liu, Y.; Wu, G. Visible and near-infrared reflectance spectroscopy—An alternative for monitoring soil contamination by heavy metals. J. Hazard. Mater. 2014, 265, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Rossel, R.V.; Walvoort, D.; McBratney, A.; Janik, L.J.; Skjemstad, J. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Stenberg, B.; Rossel, R.A.V.; Mouazen, A.M.; Wetterlind, J. Visible and near infrared spectroscopy in soil science. In Advances in Agronomy; Elsevier: Burlington, MA, USA, 2010; Volume 107, pp. 163–215. [Google Scholar]
- Bellon-Maurel, V.; Fernandez-Ahumada, E.; Palagos, B.; Roger, J.-M.; McBratney, A. Critical review of chemometric indicators commonly used for assessing the quality of the prediction of soil attributes by NIR spectroscopy. TrAC Trends Anal. Chem. 2010, 29, 1073–1081. [Google Scholar] [CrossRef]
- Song, L.; Jian, J.; Tan, D.-J.; Xie, H.-B.; Luo, Z.-F.; Gao, B. Estimate of heavy metals in soil and streams using combined geochemistry and field spectroscopy in wan-sheng mining area, Chongqing, china. Int. J. Appl. Earth Obs. Geoinf. 2015, 34, 1–9. [Google Scholar] [CrossRef]
- Thompson, A.J.B.; Hauff, P.L.; Robitaille, A.J. Alteration mapping in exploration: Application of short wave infrared (SWIR) spectroscopy. Soc. Econ. Geol. Newsl. 1999, 30, 16–27. [Google Scholar]
- Yang, K.; Browne, P.; Huntington, J.; Walshe, J. Characterising the hydrothermal alteration of the Broadlands–Ohaaki geothermal system, New Zealand, using short-wave infrared spectroscopy. J. Volcanol. Geotherm. Res. 2001, 106, 53–65. [Google Scholar] [CrossRef]
- Yang, K.; Huntington, J.F.; Browne, P.R.; Ma, C. An infrared spectral reflectance study of hydrothermal alteration minerals from the Te Mihi sector of the Wairakei geothermal system, New Zealand. Geothermics 2000, 29, 377–392. [Google Scholar] [CrossRef]
- Sun, Y.; Seccombe, P.K.; Yang, K. Application of short-wave infrared spectroscopy to define alteration zones associated with the Elura zinc–lead–silver deposit, NSW, Australia. J. Geochem. Explor. 2001, 73, 11–26. [Google Scholar] [CrossRef]
- Kerr, A.; Rafuse, H.; Sparkes, G.; Hinchey, J.; Sandeman, H. Visible/Infrared Spectroscopy (VIRS) as a Research Tool in Economic Geology: Background and Pilot Studies from Newfoundland and Labrador; Geological Survey, Report 11-1; Newfoundland and Labrador Department of Natural Resources: St. John’s, NL, Canada, 2011; pp. 145–166. [Google Scholar]
- Sonntag, I.; Laukamp, C.; Hagemann, S.G. Low potassium hydrothermal alteration in low sulfidation epithermal systems as detected by IRS and XRD: An example from the Co–O mine, Eastern Mindanao, Philippines. Ore Geol. Rev. 2012, 45, 47–60. [Google Scholar] [CrossRef]
- Zadeh, M.H.; Tangestani, M.H.; Roldan, F.V.; Yusta, I. Spectral characteristics of minerals in alteration zones associated with porphyry copper deposits in the middle part of Kerman copper belt, SE Iran. Ore Geol. Rev. 2014, 62, 191–198. [Google Scholar] [CrossRef]
- Jeong, Y.; Yu, J.; Koh, S.-M.; Heo, C.-H.; Lee, J. Spectral characteristics of minerals associated with skarn deposits: A case study of Weondong skarn deposit, South Korea. Geosci. J. 2016, 20, 167–182. [Google Scholar] [CrossRef]
- Rosso, P.H.; Pushnik, J.C.; Lay, M.; Ustin, S.L. Reflectance properties and physiological responses of Salicornia virginica to heavy metal and petroleum contamination. Environ. Pollut. 2005, 137, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.; Saleh, A.; Belal, A.; Gad, A.A. Application of near-infrared reflectance for quantitative assessment of soil properties. Egypt. J. Remote Sens. Space Sci. 2017. [Google Scholar] [CrossRef]
- Hauff, P.L. An Overview of VIS-NIR-SWIR Field Spectroscopy as Applied to Precious Metals Exploration; Spectral International Inc.: Arvada, CO, USA, 2008; Volume 80001, pp. 303–403. [Google Scholar]
- Herrmann, W.; Blake, M.; Doyle, M.; Huston, D.; Kamprad, J.; Merry, N.; Pontual, S. Short wavelength infrared (SWIR) spectral analysis of hydrothermal alteration zones associated with base metal sulfide deposits at Rosebery and Western Tharsis, Tasmania, and Highway-Reward, Queensland. Econ. Geol. Newsl. 2001, 96, 939–955. [Google Scholar] [CrossRef]
- Shin, H.; Yu, J.; Kim, J.; Yang, D.; Lee, G. Mapping the moisture content of coastal sediments using aster data for spectroscopic and mineralogical analyses: A case study in South Korea. IEEE Geosci. Remote Sens. Lett. 2015, 6, 488–497. [Google Scholar] [CrossRef]
- Shin, J.H.; Yu, J.; Kim, S.; Koh, S.-M.; Park, G. Spectral characteristics of heavy metal contaminated soils in the vicinity of Boksu mine. J. Miner. Soc. Korea 2016, 29, 89–101. [Google Scholar] [CrossRef]
- Wang, F.; Gao, J.; Zha, Y. Hyperspectral sensing of heavy metals in soil and vegetation: Feasibility and challenges. ISPRS J. Photogramm. Remote Sens. 2018, 136, 73–84. [Google Scholar] [CrossRef]
- Salminen, R.; Batista, M.; Bidovec, M.; Demetriades, A.; De Vivo, B.; De Vos, W.; Duris, M.; Gilucis, A.; Gregorauskiene, V.; Halamić, J. Geochemical Atlas of Europe, Part 1, Background Information, Methodology and Maps; Geological survey of Finland: Espoo, Finland, 2005; p. 526. [Google Scholar]
- Sipos, P.; Németh, T.; Kis, V.K.; Mohai, I. Sorption of copper, zinc and lead on soil mineral phases. Chemosphere 2008, 73, 461–469. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press: Oxford, NY, USA, 1994; p. 416. [Google Scholar]
- Vega, F.A.; Covelo, E.F.; Andrade, M. Competitive sorption and desorption of heavy metals in mine soils: Influence of mine soil characteristics. J. Colloid Interface Sci. 2006, 298, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, J.; Wu, X.; Tian, Q.; Ji, J.; Qin, Z. Possibilities of reflectance spectroscopy for the assessment of contaminant elements in suburban soils. Appl. Geochem. 2005, 20, 1051–1059. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Chen, J.; Ji, J.F.; Tian, Q.J.; Wu, X.M. Feasibility of reflectance spectroscopy for the assessment of soil mercury contamination. Environ. Sci. Technol. 2005, 39, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.Q.; Mao, Y.Q.; Ji, J.F.; Ma, H.R.; Chen, J.; Liao, Q.L. Reflectance spectroscopy study of Cd contamination in the sediments of the Changjiang River, China. Environ. Sci. Technol. 2007, 41, 3449–3454. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Liao, Q.; Ji, J. Can contaminant elements in soils be assessed by remote sensing technology: A case study with simulated data. Soil Sci. 2011, 176, 196–205. [Google Scholar] [CrossRef]
- Choe, E.; Kim, K.-W.; Bang, S.; Yoon, I.-H.; Lee, K.-Y. Qualitative analysis and mapping of heavy metals in an abandoned au–ag mine area using NIR spectroscopy. Environ. Geol. 2009, 58, 477–482. [Google Scholar] [CrossRef]
- Choe, E.; van der Meer, F.; van Ruitenbeek, F.; van der Werff, H.; de Smeth, B.; Kim, K.-W. Mapping of heavy metal pollution in stream sediments using combined geochemistry, field spectroscopy, and hyperspectral remote sensing: A case study of the Rodalquilar mining area, Se Spain. Remote Sens. Environ. 2008, 112, 3222–3233. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Zhuang, D.-F.; Singh, A.; Pan, J.-J.; Qiu, D.-S.; Shi, R.-H. Estimation of as and cu contamination in agricultural soils around a mining area by reflectance spectroscopy: A case study. Pedosphere 2009, 19, 719–726. [Google Scholar] [CrossRef]
- Ji, J.; Song, Y.; Yuan, X.; Yang, Z. Diffuse reflectance spectroscopy study of heavy metals in agricultural soils of the Changjiang River Delta, China. In Proceedings of the 19th World Congress of Soil Science, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Al Maliki, A.; Bruce, D.; Owens, G. Prediction of lead concentration in soil using reflectance spectroscopy. Environ. Technol. Innovation. 2014, 1, 8–15. [Google Scholar] [CrossRef]
- Shelton, K.L.; So, C.-S.; Chang, J.-S. Gold-rich mesothermal vein deposits of the Republic of Korea; geochemical studies of the Jungwon gold area. Econ. Geol. 1988, 83, 1221–1237. [Google Scholar] [CrossRef]
- So, C.-S.; Shelton, K.L. Stable isotope and fluid inclusion studies of gold-and silver-bearing hydrothermal vein deposits, Cheonan-Cheongyang-Nonsan mining district, Republic of Korea; Cheonan area. Econ. Geol. 1987, 82, 987–1000. [Google Scholar] [CrossRef]
- So, C.-S.; Yun, S.-T.; Shelton, K. Mesothermal gold vein mineralization of the Samdong mine, Youngdong mining district, Republic of Korea. Miner. Depos. 1995, 30, 384–396. [Google Scholar] [CrossRef]
- Choi, S.G.; Kwon, S.T.; Ree, J.H.; So, C.S.; Pak, S.J. Origin of mesozoic gold mineralization in South Korea. Isl. Arc 2005, 14, 102–114. [Google Scholar] [CrossRef]
- Choi, S.-G.; Park, S.J.; Choi, S.-H.; Shin, H.J. Mesozoic granitoids and associated gold-silver mineralization in Korea. Econ. Environ. Geol. 2001, 34, 25–38, (In Korean with English Abstract). [Google Scholar]
- Choi, S.-G.; Park, S.-J.; Kim, S.-W.; Kim, C.-S.; Oh, C.-W. Mesozoic gold-silver mineralization in South Korea: Metallogenic provinces reestimated to the geodynamic setting. Econ. Environ. Geol. 2006, 39, 567–581, (In Korean with English Abstract). [Google Scholar]
- Yoo, B.C.; Lee, H.K.; White, N.C. Mineralogical, fluid inclusion, and stable isotope constraints on mechanisms of ore deposition at the Samgwang mine (Republic of Korea)—A mesothermal, vein-hosted gold–silver deposit. Miner. Depos. 2010, 45, 161–187. [Google Scholar] [CrossRef]
- Yoo, B.-C.; Lee, G.-J.; Lee, J.-K.; Ji, E.-K.; Lee, H.-K. Element dispersion and wallrock alteration from Samgwang deposit. Econ. Environ. Geol. 2009, 42, 177–193, (In Korean with English Abstract). [Google Scholar]
- Lee, H.-G.; Choi, Y.-S. A study on the soil contamination (maps) using the handheld XRF and GIS in abandoned mining areas. J. Korean Assoc. Geogr. Inf. Stud. 2014, 17, 195–206, (In Korean with English Abstract). [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.J.; Yang, J.E. Contamination of stream and reservoir waters with arsenic from abandoned gold mine. Environ. Eng. Res. 2008, 13, 33–40. [Google Scholar] [CrossRef]
- Cho, I.-H.; Chun, S.-Y.; Chang, S.-W. Statistical assessment on the heavy metal variation in the soils around abandoned mine (case study for the Samgwang mine). J. Environ. Sci. Int. 2007, 16, 1451–1462. [Google Scholar]
- Jung, M.C. Contamination by Cd, Cu, Pb, and Zn in mine wastes from abandoned metal mines classified as mineralization types in Korea. Environ. Geochem. Health 2008, 30, 205–217, (In Korean with English Abstract). [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-R.; Lee, B.-T.; Kim, K.-W. Arsenic stabilization in mine tailings using nano-sized magnetite and zero valent iron with the enhancement of mobility by surface coating. J. Geochem. Explor. 2012, 113, 124–129. [Google Scholar] [CrossRef]
- Tenedero, R.A.; Surtida, M.B. Soil Sampling and Preparation for Laboratory Analysis; SEAFDEC Aquaculture Department: Iloilo, Philippines, 1986; p. 11. ISBN 971-8511-10-5. [Google Scholar]
- Mutanga, O.; Skidmore, A.K.; Prins, H. Predicting in situ pasture quality in the Kruger National Park, South Africa, using continuum-removed absorption features. Remote Sens. Environ. 2004, 89, 393–408. [Google Scholar] [CrossRef]
- Kokaly, R.F. Investigating a physical basis for spectroscopic estimates of leaf nitrogen concentration. Remote Sens. Environ. 2001, 75, 153–161. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Clark, R.N. Spectroscopic determination of leaf biochemistry using band-depth analysis of absorption features and stepwise multiple linear regression. Remote Sens. Environ. 1999, 67, 267–287. [Google Scholar] [CrossRef]
- Owen, T. Fundamentals of UV-Visible Spectroscopy: A Primer; Hewlett-Packard Company: Baden-Württemberg, Germany, 1996; p. 142. [Google Scholar]
- Pontual, S.; Merry, N.; Gamson, P. GMEX: Guides for Mineral Exploration: Spectral Interpretation Field Manual; AusSpec International Ltd.: Queenstown, New Zealand, 2010; Volume 1, p. 191. [Google Scholar]
- Clark, R.N.; Swayze, G.A.; Wise, R.; Livo, K.E.; Hoefen, T.; Kokaly, R.F.; Sutley, S.J. USGS Digital Spectral Library Splib06a; Digital Data Series 231; U.S. Geological Survey: Reston, VA, USA, 2007. Available online: http://speclab.cr.usgs.gov/spectral.lib06 (accessed on 22 August 2018).
- Kokaly, R.F.; Clark, R.N.; Swayze, G.A.; Livo, K.E.; Hoefen, T.M.; Pearson, N.C.; Wise, R.A.; Benzel, W.M.; Lowers, H.A.; Driscoll, R.L. USGS Spectral Library Version 7; Data Series 1035; U.S. Geological Survey: Reston, VA, USA, 2017. Available online: https://pubs.er.usgs.gov/publication/ds1035 (accessed on 22 August 2018).
- Baldridge, A.; Hook, S.; Grove, C.; Rivera, G. The aster spectral library version 2.0. Remote Sens. Environ. 2009, 113, 711–715. [Google Scholar] [CrossRef]
- Dunagan, S.C.; Gilmore, M.S.; Varekamp, J.C. Effects of mercury on visible/near-infrared reflectance spectra of mustard spinach plants (Brassica rapa P.). Environ. Pollut. 2007, 148, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Vasques, G.; Grunwald, S.; Sickman, J. Comparison of multivariate methods for inferential modeling of soil carbon using visible/near-infrared spectra. Geoderma 2008, 146, 14–25. [Google Scholar] [CrossRef]
- Nan, Z.; Zhao, C.; Li, J.; Chen, F.; Sun, W. Relations between soil properties and selected heavy metal concentrations in spring wheat (Triticum aestivum L.) grown in contaminated soils. Water Air Soil Pollut. 2002, 133, 205–213. [Google Scholar] [CrossRef]
- Tyler, G. Heavy metal pollution, phosphatase activity, and mineralization of organic phosphorus in forest soils. Soil Biol. Biochem. 1976, 8, 327–332. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Clark, R.N. Spectroscopy of rocks and minerals, and principles of spectroscopy. In Manual of Remote Sensing; Rencz, A.N., Ed.; John Wiley and Sons: New York, NY, USA, 1999; Volume 3, pp. 3–58. ISBN 0471-29405-5. [Google Scholar]
- Götze, C.; Denk, M.; Riedel, F.; Gläßer, C. Interlaboratory comparison of spectrometric laboratory measurements of a chlorite rock sample. PFG–J. Photogramm. Remote Sens. Geo-Inf. Sci. 2017, 85, 307–316. [Google Scholar] [CrossRef]
- Manning, B.A.; Goldberg, S. Adsorption and stability of arsenic (III) at the clay mineral− water interface. Environ. Sci. Technol. 1997, 31, 2005–2011. [Google Scholar] [CrossRef]
- Rybicka, E.H.; Calmano, W.; Breeger, A. Heavy metals sorption/desorption on competing clay minerals; an experimental study. Appl. Clay Sci. 1995, 9, 369–381. [Google Scholar] [CrossRef]
- Reimann, C.; Siewers, U.; Tarvainen, T.; Bityukova, L.; Eriksson, J.; Giucis, A.; Gregorauskiene, V.; Lukashev, V.K.; Matinian, N.N.; Pasieczna, A. Agricultural Soils in Northern Europe: A Geochemical Atlas; E. Schweizerbart: Stuttgart, Germany, 2003; p. 279. [Google Scholar]
- Fletcher, P.; Sposito, G. Chemical modeling of clay/electrolyte interactions of montmorillonite. Clay Miner. 1989, 24, 375–391. [Google Scholar] [CrossRef]
- Gannouni, S.; Rebai, N.; Abdeljaoued, S. A spectroscopic approach to assess heavy metals. J. Geog. Inf. Syst. 2012, 4, 242–253. [Google Scholar] [CrossRef]








| Elements | Statistics | Soil Pollution Reference | ||||
|---|---|---|---|---|---|---|
| N | Min | Max | Mean | SD | ||
| Cr | 174 | 0 | 1021 | 484 | 286 | 5 |
| Ni | 174 | 0 | 600 | 266 | 174 | 100 |
| Cu | 174 | 0 | 623 | 111 | 119 | 150 |
| Zn | 174 | 199 | 1504 | 676 | 334 | 300 |
| As | 174 | 788 | 8019 | 3208 | 1819 | 25 |
| Cd | 174 | 0 | 445 | 151 | 154 | 4 |
| Pb | 174 | 66 | 1233 | 505 | 312 | 200 |
| Site No. | Mineral Categories | ||
|---|---|---|---|
| Gangue | Rock-Forming | Alteration | |
| Sp01 | Qtz, Cal | Qtz, Ms | Ms, Phl, Dol, Clc, Ilt, Mnt |
| Sp02 | Qtz, Cal | Qtz, Ab, Ms | Ms, Phl, Dol, Clc, Ilt, Mnt, Vrm |
| Sp03 | Qtz, Cal | Qtz | Phl, Dol, Clc, Ilt, Mnt |
| Sp04 | Qtz | Qtz, Ms | Ms, Dol, Clc, Ilt |
| Sp13 | Qtz, Cal | Qtz, Ab, Ms | Ms, Phl, Clc, Ilt, Mnt |
| Sp17 | Qtz | Qtz, Ab, Ms | Ms, Phl, Dol, Clc, Ilt, Mnt |
| Sp18 | Qtz, Cal | Qtz, Ab, Ms | Ms, Phl, Dol, Clc, Ilt, Mnt |
| Major Chemical Component | Absorption Position (nm) |
|---|---|
| Fe3+ | ~900 (charge transfer slope from 750 to shorter wavelength) |
| Fe2+ | ~1000–1200 |
| -OH | ~1400 (~1550, ~1750–1850 in some minerals) |
| H2O | ~1400, ~1900, and ~1950–2100 |
| Al-OH | ~2160–2228 |
| Fe-OH | ~2230–2298 |
| Mg-OH | ~2300–2370 and ~2400 |
| Elements | Source of Variation | Sum of Squares | Degree of Freedom | Mean Sum of Squares | F | p |
|---|---|---|---|---|---|---|
| As | Between groups | 2.725 | 3 | 0.908 | 96.778 | 0.000 |
| Within groups | 1.051 | 112 | 0.009 | |||
| Total | 3.776 | 115 | - | |||
| Pb | Between groups | 0.073 | 2 | 0.037 | 106.051 | 0.000 |
| Within groups | 0.039 | 113 | 0.000 | |||
| Total | 0.112 | 115 | - |
| Elements | Wavelength (nm) | B (SE) | β | t | p | VIF | R2 | adj-R2 | Durbin-Watson | RMSEc | RMSEv |
|---|---|---|---|---|---|---|---|---|---|---|---|
| As | (coefficient) | 1.291 | 0.722 | 0.714 | 1.173 | 0.095 | 0.099 | ||||
| FDR2206 | 643.059 (209.722) | 0.322 | 3.066 | 0.003 | 4.430 | ||||||
| FDR2161 | 785.727 (177.104) | 0.334 | 4.437 | 0.000 | 2.276 | ||||||
| FDR2361 | −371.206 (92.565) | −0.321 | −4.010 | 0.000 | 2.578 | ||||||
| Pb | (coefficient) | 0.187 | 0.652 | 0.646 | 1.137 | 0.018 | 0.019 | ||||
| FDR1414 | −181.951 (32.543) | −0.518 | −5.591 | 0.000 | 2.794 | ||||||
| FDR2205 | 118.476 (33.240) | 0.330 | 3.564 | 0.001 | 2.794 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.; Yu, J.; Wang, L.; Shin, J.H. Spectral Responses of As and Pb Contamination in Tailings of a Hydrothermal Ore Deposit: A Case Study of Samgwang Mine, South Korea. Remote Sens. 2018, 10, 1830. https://doi.org/10.3390/rs10111830
Jeong Y, Yu J, Wang L, Shin JH. Spectral Responses of As and Pb Contamination in Tailings of a Hydrothermal Ore Deposit: A Case Study of Samgwang Mine, South Korea. Remote Sensing. 2018; 10(11):1830. https://doi.org/10.3390/rs10111830
Chicago/Turabian StyleJeong, Yongsik, Jaehyung Yu, Lei Wang, and Ji Hye Shin. 2018. "Spectral Responses of As and Pb Contamination in Tailings of a Hydrothermal Ore Deposit: A Case Study of Samgwang Mine, South Korea" Remote Sensing 10, no. 11: 1830. https://doi.org/10.3390/rs10111830
APA StyleJeong, Y., Yu, J., Wang, L., & Shin, J. H. (2018). Spectral Responses of As and Pb Contamination in Tailings of a Hydrothermal Ore Deposit: A Case Study of Samgwang Mine, South Korea. Remote Sensing, 10(11), 1830. https://doi.org/10.3390/rs10111830