Explaining Leaf Nitrogen Distribution in a Semi-Arid Environment Predicted on Sentinel-2 Imagery Using a Field Spectroscopy Derived Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Data Collection
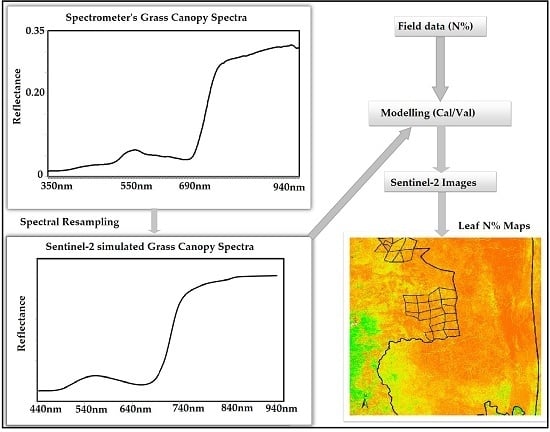
2.3. Spectral Measurements
2.4. Image Acquisition and Preprocessing
2.5. Data Analysis
2.5.1. Development of Leaf N Predictive Model
2.5.2. Explaining Leaf N Distribution
3. Results and Discussion
3.1. Descriptive Statistics
3.2. Leaf N Predictive Models
3.3. Explaining Spatial Distribution of Leaf N
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Child, R.D.; Frasier, G.W. ARS range research. Rangelands 1992, 14, 17–32. [Google Scholar]
- Friedl, M. Range condition assessment and the concept of thresholds: A viewpoint. J. Range Manag. 1991, 44, 422–426. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs, Population Division—UNPD. World Population Prospects: The 2015 Revision, Methodology of the United Nations Population Estimates and Projections; ESA/P/WP.242; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization of the United Nations). Land Degradation Assessment in Drylands (LADA). 2010. Available online: http://www.fao.org/nr/lada/ (accessed on 15 September 2017).
- Palmer, A.R.; Bennett, J.E. Degradation of communal rangelands in South Africa: Towards an improved understanding to inform policy. Afr. J. Range Forage Sci. 2013, 30, 57–63. [Google Scholar] [CrossRef]
- Grant, C.C.; Peel, M.; Zambatis, N.; van Ryssen, J.B.J. Nitrogen and phosphorus concentration in faeces: An indicator of range quality as a practical adjunct to existing range evaluation methods. Afr. J. Range Forage Sci. 2000, 17, 81–92. [Google Scholar] [CrossRef]
- Ben-Shahar, R.; Coe, M.J. The relationships between soil factors, grass nutrients and the foraging behaviour of wildebeest and zebra. Oecologia 1992, 90, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Kaszta, Z.; Marino, J.; Ramoelo, A.; Wolff, E. Bulk feeder or selective grazer: African buffalo space use patterns based on fine-scaled remotely sensed data on forage quality and quantity. Ecol. Model. 2016, 323, 115–122. [Google Scholar] [CrossRef]
- Ramoelo, A.; Skidmore, A.K.; Cho, M.A.; Mathieu, R.; Heitkönig, I.M.A.; Dudeni-Tlhone, N.; Schlerf, M.; Prins, H.H.T. Non-linear partial least square regression increases the estimation accuracy of grass nitrogen and phosphorus using in situ hyperspectral and environmental data. ISPRS J. Photogramm. Remote Sens. 2013, 82, 27–40. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Integrating imaging spectroscopy and neural networks to map grass quality in the Kruger National park, South Africa. Remote Sens. Environ. 2004, 90, 104–115. [Google Scholar] [CrossRef]
- Skidmore, A.K.; Ferwerda, J.G.; Mutanga, O.; van Wieren, S.E.; Peel, M.; Grant, R.C.; Prins, H.H.T.; Balcik, F.B.; Venus, V. Forage quality of savannas—Simultaneously mapping foliar protein and polyphenols for trees and grass using hyperspectral imagery. Remote Sens. Environ. 2010, 114, 64–72. [Google Scholar] [CrossRef]
- Knox, N.M.; Skidmore, A.K.; Prins, H.H.T.; Heitkönig, I.M.A.; Slotow, R.; van der Waal, C.; de Boer, W.F. Remote sensing of forage nutrients: Combining ecological and spectral absorption feature data. ISPRS J. Photogramm. Remote Sens. 2012, 72, 27–35. [Google Scholar] [CrossRef]
- Cho, M.A.; Skidmore, A.K. A new technique for extracting the red edge position from hyperspectral data: The linear extrapolation method. Remote Sens. Environ. 2006, 101, 181–193. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Macler, B.A.; Plummer, S.E. The effect of a red leaf pigment on the relationship between red edge and chlorophyll concentration. Remote Sens. Environ. 1991, 35, 69–76. [Google Scholar] [CrossRef]
- Ramoelo, A.; Skidmore, A.K.; Cho, M.A.; Schlerf, M.; Mathieu, R.; Heitkönig, I.M.A. Regional estimation of savanna grass nitrogen using the red-edge band of the spaceborne RapidEye sensor. Int. J. Appl. Earth Obs. Geoinf. 2012, 19, 151–162. [Google Scholar] [CrossRef]
- Ramoelo, A.; Cho, M.A.; Mathieu, R.; Madonsela, S.; van de Kerchove, R.; Kaszta, Z.; Wolff, E. Monitoring grass nutrients and biomass as indicators of rangeland quality and quantity using random forest modelling and WorldView-2 data. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 43–54. [Google Scholar] [CrossRef]
- Ramoelo, A.; Cho, M.A.; Mathieu, R.; Skidmore, A. The potential of Sentinel-2 spectral configuration to assess rangeland quality. J. Appl. Remote Sens. 2015, 9, 094096. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Red edge shift and biochemical content in grass canopies. ISPRS J. Photogramm. Remote Sens. 2007, 62, 34–42. [Google Scholar] [CrossRef]
- Huang, Z.; Turner, B.J.; Dury, S.J.; Wallis, I.R.; Foley, W.J. Estimating foliage nitrogen concentration from HYMAP data using continuum removal analysis. Remote Sens. Environ. 2004, 93, 18–29. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens. Environ. 2009, 113 (Suppl. 1), S78–S91. [Google Scholar] [CrossRef]
- Muharam, F.M.; Maas, S.J.; Bronson, K.F.; Delahunty, T. Estimating cotton nitrogen nutrition status using leaf greenness and ground cover information. Remote Sens. 2015, 7, 7007–7028. [Google Scholar] [CrossRef]
- Caturegli, L.; Casucci, M.; Lulli, F.; Grossi, N.; Gaetani, M.; Magni, S.; Bonari, E.; Volterrani, M. GeoEye-1 satellite versus ground-based multispectral data for estimating nitrogen status of turfgrasses. Int. J. Remote Sens. 2015, 36, 2238–2251. [Google Scholar] [CrossRef]
- Wang, Z.; Skidmore, A.K.; Darvishzadeh, R.; Heiden, U.; Heurich, M.; Wang, T. Leaf nitrogen content indirectly estimated by leaf traits derived from the PROSPECT model. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 3172–3182. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, T.; Darvishzadeh, R.; Skidmore, A.K.; Jones, S.; Suarez, L.; Woodgate, W.; Heiden, U.; Heurich, M.; Hearne, J. Vegetation indices for mapping canopy foliar nitrogen in a mixed temperate forest. Remote Sens. 2016, 8, 491. [Google Scholar] [CrossRef]
- Yoder, B.J.; Pettigrew-Crosby, R.E. Predicting nitrogen and chlorophyll content and concentrations from reflectance spectra (400–2500 nm) at leaf and canopy scales. Remote Sens. Environ. 1995, 53, 199–211. [Google Scholar] [CrossRef]
- Cho, M.; Skidmore, A.K.; Corsi, F.; van Wieren, S.; Sobhan, I. Estimation of green grass/herb biomass from airborne hyperspectral imagery using spectral indices and partial least square regressions. Int. J. Appl. Earth Obs. Geoinf. 2007, 9, 414–424. [Google Scholar] [CrossRef]
- Venter, F.J.; Scholes, R.J.; Eckhardt, H.C. Abiotic template and its associated vegetation pattern. In The Kruger Experience: Ecology and Management of Savanna Heterogeneity; Toit, J.T.D., Kevin, H.R., Biggs, H.C., Eds.; The Island Press: London, UK, 2003; pp. 83–129. [Google Scholar]
- Pickectt, S.T.A.; Gadenasso, M.L.; Benning, T.L. (Eds.) Biotic and Abiotic Variability as Key Determinants of Savanna Heterogeneity at Spatiotemporal Scales; Island Press: London, UK, 2003. [Google Scholar]
- Sankaran, M. Fire, grazing and the dynamics of tall-grass savannas in the Kalakad-Mundanthurai Tiger Reserve, South India. Conserv. Soc. 2005, 3, 4–25. [Google Scholar]
- Grant, C.C.; Scholes, M.C. The importance of nutrient hot-spots in the conservation and management of large wild mammalian herbivores in semi-arid savannas. Biol. Conserv. 2006, 130, 426–437. [Google Scholar] [CrossRef]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland; SANBI: Cape Town, South Africa, 2006. [Google Scholar]
- Jordan, C.F. Derivation of leaf area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.; et al. Coincident detection of crop water stress, nitrogen status and canopy density using ground-based multispectral data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Clevers, J.G.P.W.; Gitelson, A.A. Remote estimation of crop and grass chlorophyll and nitrogen content using red-edge bands on Sentinel-2 and 3. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 344–351. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves. Spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Guyot, G.; Baret, F. Utilisation de la haute résolution spectrale pour suivre l’état des couverts végétaux. In Proceedings of the 4th International Colloquium on Spectral Signatures of Objects in Remote Sensing. ESA SP-287, Assois, France, 18–22 January 1988; pp. 279–286. [Google Scholar]
- Bunke, O.; Droge, B. Bootstrap and cross-validation estimates of the prediction error for linear regression models. Ann. Stat. 1984, 12, 1400–1424. [Google Scholar] [CrossRef]
- Efron, B. Estimating the error rate of a prediction rule: Improvement on cross-validation. J. Am. Stat. Assoc. 1983, 78, 316–331. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R. Improvements on cross-validation: The 632+ Bootstrap Method. J. Am. Stat. Assoc. 1997, 92, 548–560. [Google Scholar]
- Ullah, S.; Si, Y.; Schlerf, M.; Skidmore, A.K.; Shafique, M.; Iqbal, I.A. Estimation of grassland biomass and nitrogen using MERIS data. Int. J. Appl. Earth Obs. Geoinf. 2012, 19, 196–204. [Google Scholar] [CrossRef]
- Cho, M.A.; Ramoelo, A.; Debba, P.; Mutanga, O.; Mathieu, R.; van Deventer, H.; Ndlovu, N. Assessing the effects of subtropical forest fragmentation on leaf nitrogen distribution using remote sensing data. Landsc. Ecol. 2013, 28, 1479–1491. [Google Scholar] [CrossRef]
- Loozen, Y.; Rebel, K.T.; Karssenberg, D.; Wassen, M.J.; Sardans, J.; Penuelas, J.; de Jong, S.M. Regional detection of canopy nitrogen in Mediterranean forests using the spaceborne MERIS Terrestrial Chlorophyll Index. J. Biogeosci. Discuss. 2017, 1–32. [Google Scholar] [CrossRef]
- Zengeya, F.M.; Mutanga, O.; Murwira, A. Linking remotely sensed forage quality estimates from WorldView-2 multispectral data with cattle distribution in a savanna landscape. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 513–524. [Google Scholar] [CrossRef]
- Sankaran, M.; Hanan, N.P.; Scholes, R.J.; Ratnam, J.; Augustine, A.J.; Cade, B.S.; Gignoux, J.; Higgins, S.I.; Le Roux, X.; Ludwig, F.; et al. Determinants of woody cover in African. Nature 2005, 438, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Van Wilgen, B.W. The evolution of fire management practices in savanna protected areas in South Africa. S. Afr. J. Sci. 2009, 105, 343–349. [Google Scholar] [CrossRef]
- Scholes, R.J. The influence of soil fertility on the ecology of Southern African dry savannas. J. Biogeogr. 1990, 17, 415–419. [Google Scholar] [CrossRef]







| Image No. | Reference | Dates |
|---|---|---|
| 1 | S2A_T36JUT_R092_20151226 | 16 December 2015 |
| 2 | S2A_T36JUT_R092_20160504 | 4 April 2016 |
| 3 | S2A_T36JUT_R092_20160524 | 23 April 2016 |
| 4 | S2A_T36JUT_R092_20160723 | 23 July 2016 |
| 5 | S2A_T36JUT_R092_20160812 | 12 August 2016 |
| 6 | S2A_T36JUT_R092_20160822 | 22 August 2016 |
| 7 | S2A_T36JUT_R092_20160901 | 1 September 2016 |
| 8 | S2A_T36JUT_R092_20160921 | 21 September 2016 |
| 9 | S2A_T36JUT_R092_20161011 | 11 October 2016 |
| Min | Max | Mean | CV (%) | N |
|---|---|---|---|---|
| 0.54 | 2.05 | 0.88 | 36.35 | 30 |
| Variables | R2 | RMSE (%N) | RRMSE (%) | p < 0.05 |
|---|---|---|---|---|
| 443 nm | 0.11 | 0.31 | 35.23 | No |
| 490 nm | 0.14 | 0.30 | 34.09 | Yes |
| 560 nm | 0.01 | 0.32 | 36.36 | No |
| 665 nm | 0.27 | 0.28 | 31.82 | Yes |
| 705 nm | 0.15 | 0.30 | 34.09 | Yes |
| 740 nm | 0.23 | 0.29 | 32.95 | Yes |
| 783 nm | 0.30 | 0.27 | 30.68 | Yes |
| 842 nm | 0.27 | 0.28 | 31.82 | Yes |
| REP | 0.70 | 0.18 | 20.45 | Yes |
| NDVI | 0.59 | 0.21 | 23.86 | Yes |
| NDVI1 | 0.60 | 0.21 | 23.86 | Yes |
| NDVI_RE1 | 0.59 | 0.21 | 23.86 | Yes |
| NDVI_RE2 | 0.51 | 0.23 | 26.14 | Yes |
| NDVI_RE3 | 0.69 | 0.18 | 20.45 | Yes |
| NDVI_RE4 | 0.73 | 0.17 | 19.32 | Yes |
| NDVI_RE5 | 0.69 | 0.18 | 20.45 | Yes |
| NDVI_RE6 | 0.22 | 0.29 | 32.95 | Yes |
| NDVI_RE7 | 0.68 | 0.19 | 22.00 | Yes |
| SR | 0.70 | 0.18 | 20.45 | Yes |
| SR1 | 0.70 | 0.18 | 20.45 | Yes |
| SR_RE1 | 0.68 | 0.18 | 20.45 | Yes |
| SR_RE2 | 0.56 | 0.22 | 25.00 | Yes |
| SR_RE3 | 0.75 | 0.17 | 19.32 | Yes |
| SR_RE4 | 0.74 | 0.17 | 19.32 | Yes |
| SR_RE5 | 0.74 | 0.17 | 19.32 | Yes |
| SR_RE6 | 0.22 | 0.29 | 32.95 | Yes |
| SR_RE7 | 0.72 | 0.17 | 19.32 | Yes |
| CIred edge | 0.75 | 0.17 | 19.32 | Yes |
| CIgreen | 0.69 | 0.18 | 20.45 | Yes |
| MTCI | 0.75 | 0.17 | 19.32 | Yes |
| F-Statistics | Significance Level (p < 0.05) | |
|---|---|---|
| Geo vs. STDEV N (%) | F(10,610) = 5.4770 | yes |
| Geo vs. Mean N (%) | F(10,610) = 3.8157 | yes |
| Geo vs. Median N (%) | F(10,610) = 2.0364 | yes |
| Fire Freq vs. STDEV N (%) | F(9611) = 5.0712 | yes |
| Fire Freq vs. Mean N (%) | F(9611) = 6.1198 | yes |
| Fire Freq vs. Median N (%) | F(9611) = 4.8006 | yes |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramoelo, A.; Cho, M.A. Explaining Leaf Nitrogen Distribution in a Semi-Arid Environment Predicted on Sentinel-2 Imagery Using a Field Spectroscopy Derived Model. Remote Sens. 2018, 10, 269. https://doi.org/10.3390/rs10020269
Ramoelo A, Cho MA. Explaining Leaf Nitrogen Distribution in a Semi-Arid Environment Predicted on Sentinel-2 Imagery Using a Field Spectroscopy Derived Model. Remote Sensing. 2018; 10(2):269. https://doi.org/10.3390/rs10020269
Chicago/Turabian StyleRamoelo, Abel, and Moses Azong Cho. 2018. "Explaining Leaf Nitrogen Distribution in a Semi-Arid Environment Predicted on Sentinel-2 Imagery Using a Field Spectroscopy Derived Model" Remote Sensing 10, no. 2: 269. https://doi.org/10.3390/rs10020269
APA StyleRamoelo, A., & Cho, M. A. (2018). Explaining Leaf Nitrogen Distribution in a Semi-Arid Environment Predicted on Sentinel-2 Imagery Using a Field Spectroscopy Derived Model. Remote Sensing, 10(2), 269. https://doi.org/10.3390/rs10020269