1. Introduction
Yellow rust (
Puccinia striiformis) is one of the most severe epidemic diseases for winter wheat in China, affecting more than 6.7 million ha during 2000–2016 (
http://cb.natesc.gov.cn/sites/cb/). Under prolonged stress, crop growth and productivity are impaired [
1]. Due to the high costs of chemical control, a real-time nondestructive detection of the pathological progression of rust on leaves is vital for effective management using precision agriculture. Currently, hyperspectral analyses are the main approach for detecting foliar biophysical variations, and provide the basis for tracking rust development in hyperspectral language [
2].
The interaction of electromagnetic radiation with plant leaves is governed by their biophysical constituents and response to infestations [
3,
4,
5]. Numerous researches have been undertaken in order to understand these host–pathogen interactions from the hyperspectral perspective [
3,
6], Most of them attempted to link vegetation indices (VIs), which employ algebraic combination on specific spectral bands with specific foliar constituent [
7,
8]. For instance, Mahlein, et al. [
9] analyzed the hyperspectral signatures of cercospora leaf spot, sugar beet rust and powdery mildew on sugar beet plants, and developed specific vegetation indices to detect and identify various diseases with an overall accuracy of 88.3%. Shi, et al. [
10] tested a total of 18 typical spectral features for the classification of yellow rust, powdery mildew, and aphid on wheat. The results showed the potential of VIs-based kernel discriminant analysis (KDA) for detecting various diseases under complicated farmland circumstances.
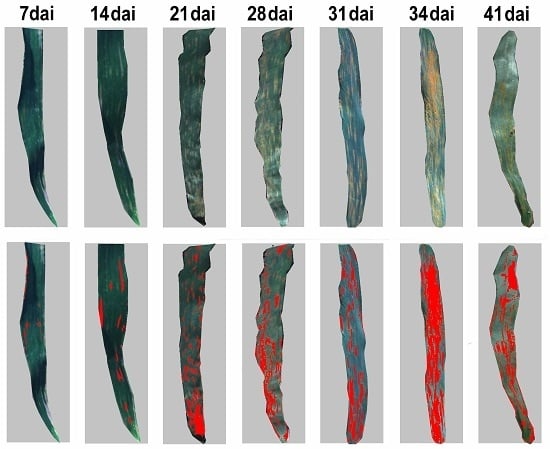
Pathologically, the development of yellow rust comprises five spore stages, uredospores, appressorium, basidiospores, spermatia, and aeciospores. The foliar biophysical variations are critical indicators for tracking the progression of host–pathogen interactions through the different stages. In the initial two stages, uredospores and appressorium develop on the upper side of leaves with random scatter distribution of pustules that are invisible to the naked eye [
11]. Subsequently, basidiospores, spermatia and aeciospores grow hyphae and haustorium inside the cellular tissue of leaves, and induce a series of biophysical lesions [
12]. Currently, the majority of studies on agricultural diseases monitoring, using earth observation, have focused on a given infestation stage, usually late in the progression of the disease [
6,
13,
14,
15,
16,
17]. Although focusing on late stages of the infestations might maximize the discriminant power of the methods, the outcomes will be less relevant for crop protection as the damage will be detected too late for any efficient actions. Thus, further effort is required to develop techniques that could track the early development of a host–pathogen interaction such as yellow rust on wheat.
The development of rust infestation is a complicated process, which is hard to characterize using the preexisting spectral features and methods. The availability of hyperspectral continuum observations may facilitate the detection of the host–pathogen processes within entire epidemic stages of yellow rust on wheat. Nevertheless, tracking the progress of the infestation well still be affected by the following aspects: (1) the pre-existing VIs are not disease-specific; (2) these VIs might vary non-linearly in relation to the increase of pathogen incidence representing poorly the variation in the spectral signature of the disease process; (3) spatial and spectral redundancy have to be taken into account. The continuous wavelet transformation (CWT) has been proven to be a promising tool to capture subtle spectral absorption characteristics in detection of foliar constituents [
18,
19,
20]. The CWT-derived wavelet features are capable of decomposing raw spectral data into different amplitudes and scales (frequencies) in order to facilitate the recognition of subtle variation (or signals) and the potential on retrieving foliar constituents [
21,
22,
23,
24,
25].
While the wavelet-based technique has been used in hyperspectral analysis, the mechanism of the CWT-derived spectral features for tracking yellow rust development still remains unclear. Furthermore, the ideal spectral features and models for tracking progressive host–pathogen interaction of yellow rust are expected to have not only high sensitive to the foliar biophysical parameters, but also robustness for different infestation stages and various sensors. Therefore, this study aimed: (1) to identify a wavelet-based rust sensitive feature set (WRSFs) for characterizing the spectral changes caused by yellow rust infestation at different stages; (2) to provide insight of the proposed WRSFs into specific leaf biophysical variations in the yellow rust development progress; (3) to evaluate the performance of the proposed WRSFs as input feature space for tracking yellow rust progress and retrieving rust severities using continuous multitemporal hyperspectral observation covering the entire circle of yellow rust infestation.
4. Discussion
To our knowledge, this work may be the first attempt to track progressive host–pathogen interaction in the continuous hyperspectral observations. By using the wavelet-based feature extraction approach, a series of independent wavelet spectral features were extracted to characterize foliar biophysical dynamics caused by yellow rust infestation. In the process of CWT, the Mexican Hat mother wavelet fitted well with the absorption features of the original spectral responses of rust infestation, and the best capturing of changes on shape of spectral signals caused by the infestation development could be achieved by changing scale factors. In this study, CWT was implemented for 10 scales. The wavelet signatures at the scales of 2–5 retained a large amount of basic information of the original spectral reflectance and were useful at providing reliable responses pertaining to disease detection. These findings are also in agreement with the research by Zhang et al. [
18].
The identified WRSFs performed well in characterizing these progressive spectral responses of foliar biophysical changes caused by rust infestation (
Figure 5). The ANOVA and univariate correlation analysis suggest that variations in foliar biophysical parameters induced by rust, such as chlorophyll content, anthocyanin content, nutrients, and dry matter accumulation, are best described by combining the extracted WRSFs from the hyperspectral perspective. Specifically, anthocyanin is the first detectable pigment induced by foliar stresses, and the increase of anthocyanin content can be detected by the WF03 between 573–584 nm. Additionally, the pathogens that attack various organs and tissues on leaves produce effects on plant structure and dry matter accumulation. The wavelet coefficients at the region of 478–496 nm, 683–697 nm, and 739–761 nm in the process of rust infestation prove that the impact of rust infestation on dry matter content and the upward movement of nitrogen could be detected by combining the WF01, WF04, and WF05. Meanwhile, pathogens do interfere with photosynthesis by affecting the chloroplasts and cause their degeneration, which are presented by the wavelet coefficient decrease of WF02 at 535–548 nm. This finding is also consistent with Sawut et al. [
41]’s study, which reported the yellow bands near 550 nm are sensitive to photosynthetic pigments and have great potential in early plant stress detection.
The PLSR models have been successfully used for DR estimations in the progression of yellow rust infestation. The results show that the WRSFs-PLSR model outperformed the VIs-PLSR on rust retrieving. More importantly, compared with VIs-PLSR, the WRSFs-PLSR provides apt pathological and biophysical information in the DR estimation procedure based on the VIP method. The analysis of importance in WRSFs measured by VIP scores has shown that the highest score structures for the early inoculation stage (before 14th dai) were contributed by WF02 and WF03 which are sensitive to CHL and ANTH. Additionally, as the symptoms became visible (after 21st dai), WF01 and WF04 which respond to PDM and NBI, showed higher importance in the model fitness. Finally, in the mid–late stage (28th–34th dai), because the rust spores rolled and ruptured the foliar epidermis, the importance of WF05, which is sensitive to the foliar structure rapidly increased. These importance dynamics in VIP scores during the PLSR analysis are consistent with the pathological progress of rust reported in related researches [
7,
12,
51,
52]. Furthermore, it was noteworthy that, in many cases, the shape-based wavelet features had been proven have greater robustness than VIs features. For example, Zhang, et al.’s study reported that, compared with traditional VIs or other spectral features, such as first-order derivative spectral and continuum removal features, no normalization procedure is needed for the application of the wavelet features, which makes them more robust in dealing with noises interferences [
53]. This finding also partially supports our new proposed WRSFs that was more notable robust and sensitive to the foliar biophysical dynamics caused by the progressive infestation of yellow rust comparing with traditional VIs.
The efficiency of each kind of feature space in tracking rust lesions was evaluated using the identified WRSFs and VIs as input feature space in LDA and SVM classification methods. Compared to the LDA with empirical risk minimization, structural risk minimization-based SVMs proved to be the most instrumental strategy for automatic differentiation between healthy leaves and leaves infested with yellow rust, especially in the pre-symptomatic stages. In addition, compared with the LDA frame, the kernel-based SVM classifier performed better in limited the effect of high entropy on the sample set, especially before the visible symptoms occurred on the leaves, which achieved accuracies 3.5–6.4% higher than the LDA classification in yellow rust detection in different measurement dai (
Figure 7). These conclusions are also in agreement with previous studies [
42,
47]. More importantly, compared with the VIs-based feature space, the greater orthogonality of the feature space produced by the WRSFs resulted in a more stable margin hyperplane for separation. This enhanced the information content and increased the generalization ability of SVM in tracking rust development at different stages. Unlike the VIs which use ratios of two or three bands of the visible- and near infrared region to normalize variations in the magnitude of reflectance, the shape-based wavelet spectral features produced by CWT with optimal combinations of positions and scales are able to capture the comprehensive biophysical variations and spectral responses caused by disease, such as the chlorophyll concentration variations and corresponding “blue shifting” phenomenon in spectral domain [
20,
54]. This characteristic explains why the WRSFs-based model outperform the VIs for rust detection and differentiation. Our findings also suggest that, by using the WRSFs-based SVM model, the earliest detection of yellow rust infestation can be achieved in 7th dai, with the acceptable accuracies of 67.4% and 86.5% for ASD and Headwall measurements.
Comparing the spectral datasets of yellow rust collected in this study, there is much more noise in the detection of foliar symptoms in the non-imaging data obtained by the ASD spectroradiometer in comparison to the pixel-based spectral images collected by the Headwall spectrograph. The main explanation to this finding is that the spectral signatures measured by the spectroradiometer are the mean of the reflectance of both healthy and diseased plant tissues in single leaves. With imaging sensor systems, such as the Headwall spectrograph, a pixel-wise attribution of rust lesions and tissue is used. Therefore, the comparison of classification accuracy between the non-imaging and imaging datasets proven that the hyperspectral imaging system enabled detection of the small size of rust colonies, especially for the improvement of early disease monitoring. Meanwhile, because the proposed WRSFs include only the most relevant information of the infestation of yellow rust, the WRSFs-based models have better capacity of reducing the computational burden and achieving a near-real time diseases detection. These findings will lead to a better understanding of the pathogen–host interactions of yellow rust in field from the perspective of spectrum. However, although the inclusion of our findings can be carried out to indicate the pathologically related foliar lesion, the yellow rust-specific canopy structure characteristics were not explored in current study. Future work will evaluate the canopy structure effect on the contextual findings in order to conduct an operational application on UVA or space platform. Therefore, in this study, there were no additional nondestructive measurements in the field used for operational application.