Difference and Potential of the Upward and Downward Sun-Induced Chlorophyll Fluorescence on Detecting Leaf Nitrogen Concentration in Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Measurements of Sun-Induced Fluorescence at the Leaf Scale
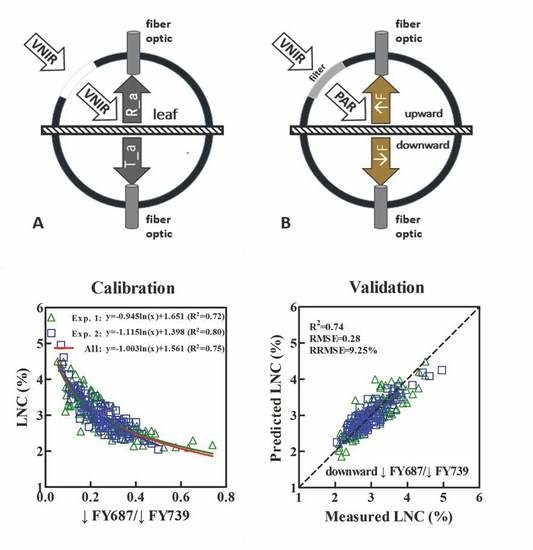
2.2.1. Acquisition of the Upward (↑F) and Downward (↓F) SIF Spectra at the Leaf Scale
2.2.2. Sun-Induced Fluorescence (SIF) Yield Indices
2.3. Measurements of Leaf Biochemical Parameters
2.4. Calculation of Vegetation Indices
2.5. Statistical Analysis
3. Results
3.1. Characteristics of SIF Spectra at the Leaf Scale under Varied Nitrogen Rates
3.2. Correlations between the Upward and Downward SIF Yield and Three Given LNC Ranges for the Winter Wheat
3.3. Constructing the LNC Estimation Models on SIF Yield Indices in Wheat
3.4. Validation of the Estimated LNC Model on SIF Yield Indices in Wheat
3.5. Assessing the LNC Models on SIF Yield Indices under Individual Stage, Different LNC, Chl Content, and Leaf Structure LMA
4. Discussion
4.1. Power of the Upward and Downward SIF Yield Indices (↑FY and ↓FY) in LNC Detection
4.2. Reason for Better Performance of Peak Ratio Indices in LNC Detection
4.3. Performance of the Relationships between SIF Yield Indices and LNC under the Varied Chl Content and Leaf Structure LMA
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clevers, J.; Gitelson, A.A. Remote estimation of crop and grass chlorophyll and nitrogen content using red-edge bands on Sentinel-2 and -3. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 344–351. [Google Scholar] [CrossRef]
- Miao, Y.; Mulla, D.J.; Hernandez, J.A.; Wiebers, M.; Robert, P.C. Potential impact of precision nitrogen management on corn yield, protein content, and test weight. Soil Sci. Soc. Am. J. 2007, 71, 1490–1499. [Google Scholar] [CrossRef]
- Diacono, M.; Rubino, P.; Montemurro, F. Precision nitrogen management of wheat: A review. Agron. Sustain. Dev. 2013, 33, 219–241. [Google Scholar] [CrossRef]
- Yang, J.; Gong, W.; Shi, S.; Du, L.; Sun, J.; Song, S. Estimation of nitrogen content based on fluorescence spectrum and principal component analysis in paddy rice. Plamt Soil Environ. 2016, 62, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.; Sakaiya, E.; Zhu, Y.; Takahashi, W. Diagnostic mapping of canopy nitrogen content in rice based on hyperspectral measurements. Remote Sens. Environ. 2012, 126, 210–221. [Google Scholar] [CrossRef]
- Yao, X.; Ren, H.; Cao, Z.; Tian, Y.; Cao, W.; Zhu, Y.; Cheng, T. Detecting leaf nitrogen content in wheat with canopy hyperspectrum under different soil backgrounds. Int. J. Appl. Earth Obs. Geoinf. 2014, 32, 114–124. [Google Scholar] [CrossRef]
- Yao, X.; Huang, Y.; Shang, G.; Zhou, C.; Cheng, T.; Tian, Y.; Cao, W.; Zhu, Y. Evaluation of Six Algorithms to Monitor Wheat Leaf Nitrogen Concentration. Remote Sens. 2015, 7, 14939–14966. [Google Scholar] [CrossRef] [Green Version]
- Schlemmer, M.; Gitelson, A.A.; Schepers, J.; Ferguson, R.; Peng, Y.; Shanahan, J.; Rundquist, D. Remote estimation of nitrogen and chlorophyll contents in maize at leaf and canopy levels. Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Haboudane, D.; Tremblay, N.; Wang, J.; Vigneault, P.; Li, B. New spectral indicator assessing the efficiency of crop nitrogen treatment in corn and wheat. Remote Sens. Environ. 2010, 114, 1987–1997. [Google Scholar] [CrossRef] [Green Version]
- Nguy-Robertson, A.; Gitelson, A.A.; Peng, Y.; Viña, A.; Arkebauer, T.; Rundquist, D. Green Leaf Area Index Estimation in Maize and Soybean: Combining Vegetation Indices to Achieve Maximal Sensitivity. Agron. J. 2012, 104, 1336–1347. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Kooistra, L. Using hyperspectral remote sensing data for retrieving canopy chlorophyll and nitrogen content. IEEE J. Sel. Top. Earth Obs. Remote Sens. 2012, 5, 574–583. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators: A review. Agron. Sustain. Dev. 2011, 32, 451–464. [Google Scholar] [CrossRef]
- Van Wittenberghe, S.; Alonso, L.; Verrelst, J.; Moreno, J.; Samson, R. Bidirectional sun-induced chlorophyll fluorescence emission is influenced by leaf structure and light scattering properties—A bottom-up approach. Remote Sens. Environ. 2015, 158, 169–179. [Google Scholar] [CrossRef]
- Govindjee, G. Chlorophyll Fluorescence: A Bit of Basics and History; Springer: Dordrecht, The Netherlands, 2004; pp. 1–41. [Google Scholar]
- Kuckenberg, J.; Tartachnyk, I.; Noga, G. Detection and differentiation of nitrogen-deficiency, powdery mildew and leaf rust at wheat leaf and canopy level by laser-induced chlorophyll fluorescence. Biosyst. Eng. 2009, 103, 121–128. [Google Scholar] [CrossRef]
- Cendrero-Mateo, M.P.; Moran, M.S.; Papuga, S.A.; Thorp, K.R.; Alonso, L.; Moreno, J.; Ponce-Campos, G.; Rascher, U.; Wang, G. Plant chlorophyll fluorescence: Active and passive measurements at canopy and leaf scales with different nitrogen treatments. J. Exp. Bot. 2016, 67, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by invivo, chlorophyll a, fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Živčák, M.; Olšovská, K.; Slamka, P.; Galambošová, J.; Rataj, V.; Shao, H.B.; Brestič, M. Application of chlorophyll fluorescence performance indices to assess the wheat photosynthetic functions influenced by nitrogen deficiency. Plant Soil Environ. 2014, 60, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Živčák, M.; Olšovská, K.; Slamka, P.; Galambošová, J.; Rataj, V.; Shao, H.B.; Kalaji, H.M.; Brestič, M. Measurements of chlorophyll fluorescence in different leaf positions may detect nitrogen deficiency in wheat. Zemdirbyste-Agriculture 2014, 101, 437–444. [Google Scholar] [CrossRef]
- Yang, J.; Gong, W.; Shi, S.; Du, L.; Sun, J.; Song, S.; Chen, B.; Zhang, Z. Analyzing the performance of fluorescence parameters in the monitoring of leaf nitrogen content of paddy rice. Sci. Rep. 2016, 6, 28787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.L.; Barbottin, A.; Jeuffroy, M.H.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crops Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Buschmann, C. Variability and application of the chlorophyll fluorescence emission ratio red/far-red of leaves. Photosynth. Res. 2007, 92, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.; Buschmann, C.; Lichtenthaler, H.K. The chlorophyll fluorescence ratio F735/F700 as an accurate measure of the chlorophyll content in plants. Remote Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Hak, R.; Rinderle, U. The chlorophyll fluorescence ratio F690/F730 in leaves of different chlorophyll content. Photosynth. Res. 1990, 25, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Rosema, A.; Zahn, H. Laser Pulse Energy Requirements for Remote Sensing of Chlorophyll Fluorescence. Remote Sens. Environ. 1997, 62, 101–108. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhao, C.J.; Liu, L.Y.; Wang, J.H.; Wang, R.C. Chlorophyll Fluorescence Detected Passively by Difference Reflectance Spectra of Wheat (Triticum aestivum L.) Leaf. J. Integr. Plant Biol. 2005, 47, 1228–1235. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjarvi, E.; Atherton, J.; Van der Tol, C.; Flexas, J.; Pfundel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef] [PubMed]
- Rascher, U.; Alonso, L.; Burkart, A.; Cilia, C.; Cogliati, S.; Colombo, R.; Damm, A.; Drusch, M.; Guanter, L.; Hanus, J.; et al. Sun-induced fluorescence—A new probe of photosynthesis: First maps from the imaging spectrometer HyPlant. Glob. Chang. Biol. 2015, 21, 4673–4684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papageorgiou, G.C.; Govindjee, G. Chlorophyll a Fluorescence—A Signature of Photosynthesis; Springer: Dordrecht, The Netherlands, 2004; p. 818. [Google Scholar]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Franck, F.; Juneau, P.; Popovic, R. Resolution of the Photosystem I and Photosystem II contributions to chlorophyll fluorescence of intact leaves at room temperature. BBA Bioenerg. 2002, 1556, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Guanter, L.; Zhang, Y.; Jung, M.; Joiner, J.; Voigt, M.; Berry, J.A.; Frankenberg, C.; Huete, A.R.; Zarco-Tejada, P.; Lee, J.E.; et al. Global and time-resolved monitoring of crop photosynthesis with chlorophyll fluorescence. Proc. Natl. Acad. Sci. USA 2014, 111, E1327–E1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Zhang, Y.; Jiao, Q.; Peng, D. Assessing photosynthetic light-use efficiency using a solar-induced chlorophyll fluorescence and photochemical reflectance index. Int. J. Remote. Sens. 2013, 34, 4264–4280. [Google Scholar] [CrossRef]
- Wagle, P.; Zhang, Y.; Jin, C.; Xiao, X. Comparison of solar-induced chlorophyll fluorescence, light-use efficiency, and process-based GPP models in maize. Ecol. Appl. 2016, 26, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Liu, Z.; Huo, H.; Li, Z.L.; Nerry, F.; Wang, Q.; Li, X. Early Water Stress Detection Using Leaf-Level Measurements of Chlorophyll Fluorescence and Temperature Data. Remote Sens. 2015, 7, 3232–3249. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Guo, Y.; Huang, Y.; Reddy, K.N.; Zhao, Y.; Molin, W.T. Detection of the onset of glyphosate-induced soybean plant injury through chlorophyll fluorescence signal extraction and measurement. J. Appl. Remote Sens. 2015, 9, 097098. [Google Scholar] [CrossRef]
- Tubuxin, B.; Rahimzadeh-Bajgiran, P.; Ginnan, Y.; Hosoi, F.; Omasa, K. Estimating chlorophyll content and photochemical yield of photosystem II (PhiPSII) using solar-induced chlorophyll fluorescence measurements at different growing stages of attached leaves. J. Exp. Bot. 2015, 66, 5595–5603. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Liu, L.; Liu, X.; Hu, J. Response of Canopy Solar-Induced Chlorophyll Fluorescence to the Absorbed Photosynthetically Active Radiation Absorbed by Chlorophyll. Remote Sens. 2017, 9, 911. [Google Scholar] [CrossRef]
- Zhang, Y.; Guanter, L.; Berry, J.A.; Van der Tol, C.; Yang, X.; Tang, J.; Zhang, F. Model-based analysis of the relationship between sun-induced chlorophyll fluorescence and gross primary production for remote sensing applications. Remote Sens. Environ. 2016, 187, 145–155. [Google Scholar] [CrossRef]
- Louis, J.; Cerovic, Z.G.; Moya, I. Quantitative study of fluorescence excitation and emission spectra of bean leaves. J. Photochem. Photobiol. B 2006, 85, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.; Gomez-Chova, L.; Vila-Frances, J.; Amoros-Lopez, J.; Guanter, L.; Calpe, J.; Moreno, J. Sensitivity analysis of the fraunhofer line discrimination method for the measurement of chlorophyll fluorescence using a field spectroradiometer. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Barcelona, Spain, 23–28 July 2007; pp. 3756–3759. [Google Scholar]
- Van Wittenberghe, S.; Alonso, L.; Verrelst, J.; Hermans, I.; Delegido, J.; Veroustraete, F.; Valcke, R.; Moreno, J.; Samson, R. Upward and downward solar-induced chlorophyll fluorescence yield indices of four tree species as indicators of traffic pollution in Valencia. Environ. Pollut. 2013, 173, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cheng, T.; Jia, M.; Zhou, K.; Lu, N.; Yao, X.; Tian, Y.; Zhu, Y.; Cao, W. PROCWT: Coupling PROSPECT with continuous wavelet transform to improve the retrieval of foliar chemistry from leaf bidirectional reflectance spectra. Remote Sens. Environ. 2018, 206, 1–14. [Google Scholar] [CrossRef]
- Rouse, J.W. Monitoring the Vernal Advancement and Retrogradation (Greenwave Effect) of Natural Vegetation; NASA/GSFCT Technical Report; NTRS: Chicago, IL, USA, 1974.
- Jiang, Z.; Huete, A.; Didan, K.; Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Guyot, G.; Baret, F. Utilisation de la Haute Resolution Spectrale pour Suivre L’etat des Couverts Vegetaux. Spectr. Signat. Objects Remote Sens. 1988, 287, 279. [Google Scholar]
- Gilabert, M.A.; Gandía, S.; Meliá, J. Analyses of spectral-biophysical relationships for a corn canopy. Remote Sens. Environ. 1996, 55, 11–20. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Cheng, T.; Riaño, D.; Ustin, S.L. Detecting diurnal and seasonal variation in canopy water content of nut tree orchards from airborne imaging spectroscopy data using continuous wavelet analysis. Remote Sens. Environ. 2014, 143, 39–53. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Miao, Y.; Yuan, F.; Ustin, S.L.; Yu, K.; Yao, Y.; Huang, S.; Bareth, G. Hyperspectral canopy sensing of paddy rice aboveground biomass at different growth stages. Field Crops Res. 2014, 155, 42–55. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Huang, Y.; Verhoef, W.; Van der Tol, C.; Dai, B.; Liu, L.; Zhao, H.; Liu, G. Quantitative Estimation of Fluorescence Parameters for Crop Leaves with Bayesian Inversion. Remote Sens. 2015, 7, 14179–14199. [Google Scholar] [CrossRef] [Green Version]
- Vogelmann, T.C.; Han, T. Measurement of gradients of absorbed light in spinach leaves from chlorophyll fluorescence profiles. Plant Cell Environ. 2000, 23, 1303–1311. [Google Scholar] [CrossRef] [Green Version]
- Vogelman, T.C.; Nishio, J.N.; Smith, W.K. Leaves and light capture: Light propagation and gradients of carbon fixation within leaves. Trends Plant Sci. 1996, 1, 65–70. [Google Scholar] [CrossRef]
- Thomas, J.R.; Gausman, H.W. Leaf Reflectance vs. Leaf Chlorophyll and Carotenoid Concentrations for Eight Crops1. Agron. J. 1977, 69, 799–802. [Google Scholar] [CrossRef]
- Bauerle, W.L.; Weston, D.J.; Bowden, J.D.; Dudley, J.B.; Toler, J.E. Leaf absorptance of photosynthetically active radiation in relation to chlorophyll meter estimates among woody plant species. Sci. Hortic. 2004, 101, 169–178. [Google Scholar] [CrossRef]
- Wang, J.F.; He, D.X.; Song, J.X.; Dou, H.J.; Du, W.F. Non-destructive measurement of chlorophyll in tomato leaves using spectral transmittance. Int. J. Agric. Biol. Eng. 2015, 8, 73–78. [Google Scholar]
- Fournier, A.; Daumard, F.; Champagne, S.; Ounis, A.; Goulas, Y.; Moya, I. Effect of canopy structure on sun-induced chlorophyll fluorescence. ISPRS J. Photogramm. Remote Sens. 2012, 68, 112–120. [Google Scholar] [CrossRef]
- Middleton, E.M.; Cheng, Y.B.; Corp, L.A.; Campbell, P.K.E.; Huemmrich, K.F.; Zhang, Q.; Kustas, W.P. Canopy level Chlorophyll Fluorescence and the PRI in a cornfield. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Munich, Germany, 22–27 July 2012; pp. 7117–7120. [Google Scholar]
- Butler, W.L.; Kitajima, M. Energy transfer between Photosystem II and Photosystem I in chloroplasts. Biochim. Biophys. Acta 1975, 396, 72–85. [Google Scholar] [CrossRef]
- Hák, R.; Lichtenthaler, H.K.; Rinderle, U. Decrease of the chlorophyll fluorescence ratio F690/F730 during greening and development of leaves. Radiat. Environ. Biophys. 1990, 29, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Pedrós, R.; Goulas, Y.; Jacquemoud, S.; Louis, J.; Moya, I. FluorMODleaf: A new leaf fluorescence emission model based on the PROSPECT model. Remote Sens. Environ. 2010, 114, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Rossini, M.; Meroni, M.; Celesti, M.; Cogliati, S.; Julitta, T.; Panigada, C.; Rascher, U.; Van der Tol, C.; Colombo, R. Analysis of Red and Far-Red Sun-Induced Chlorophyll Fluorescence and Their Ratio in Different Canopies Based on Observed and Modeled Data. Remote Sens. 2016, 8, 412. [Google Scholar] [CrossRef]
- Errecart, P.M.; Agnusdei, M.G.; Lattanzi, F.A.; Marino, M.A. Leaf nitrogen concentration and chlorophyll meter readings as predictors of tall fescue nitrogen nutrition status. Field Crops Res. 2012, 129, 46–58. [Google Scholar] [CrossRef]
- Li, J.W.; Zhang, J.X.; Zhao, Z.; Lei, X.D.; Xu, X.L.; Lu, X.X.; Weng, D.L.; Gao, Y.; Cao, L.K. Use of fluorescence-based sensors to determine the nitrogen status of paddy rice. J. Agric. Sci. 2013, 151, 862–871. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Hennig, S.D.; Gnyp, M.L.; Chen, X.P.; Jia, L.L.; Bareth, G. Evaluating hyperspectral vegetation indices for estimating nitrogen concentration of winter wheat at different growth stages. Precis. Agric. 2010, 11, 335–357. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]












| Experiment (Exp.) | Year | Plot Size (m × m) | Wheat Cultivar | Planting Density | N Application Rate (Kg·ha−1) | Sampling Date | Number of Samples |
|---|---|---|---|---|---|---|---|
| 1 Rugao (32°15′N, 120°38′E) | 2016–2017 | 5 × 6 | Yangmai 15 (V1) Yangmai 16 (V2) | 25 cm 40 cm | 0, 150, 300 | Jointing, | 29 |
| Booting, | 30 | ||||||
| Heading, | 30 | ||||||
| Anthesis | 30 | ||||||
| 2 Sihong (33°27′N, 118°13′E) | 2016–2017 | 6 × 7 | Huaimai 20 (V3) Xumai 30 (V4) | 25 cm | 0, 90, 180, 270, 360 | Booting, | 30 |
| Heading, | 30 | ||||||
| Anthesis | 30 |
| SIF Yield Indices | Definition | Formula | |
|---|---|---|---|
| Upward | ↑FY687 (%) | Upward SIF emission at 687 nm normalized by APAR | ↑F687/APAR |
| ↑FY739 (%) | Upward SIF emission at 739 nm normalized by APAR | ↑F739/APAR | |
| ↑FY687/↑FY739 (%) | The ratio of upward SIF emission peaks | ↑FY687/↑FY739 | |
| Downward | ↓FY687 (%) | Downward SIF emission at 687 nm normalized by APAR | ↓F687/APAR |
| ↓FY739 (%) | Downward SIF emission at 739 nm normalized by APAR | ↓F739/APAR | |
| ↓FY687/↓FY739 (%) | The ratio of downward SIF emission peaks | ↓FY687/↓FY739 |
| Index | Equation | Reference |
|---|---|---|
| Normalized difference vegetation index (NDVI) | (R810 − R690)/(R810 + R690) | [44] |
| Enhanced vegetation index (EVI2) | 2.5 × (R810 − R690)/(R810 + 2.4 × R690 + 1) | [45] |
| Red edge inflection point (REP) | R700 + 40 × [(R670 + R780)/2 − R700)/(R740 − R700)] | [46] |
| Green NDVI | (R800 − R550)/(R800 + R550) | [47] |
| Green chlorophyll index (CIgreen) | (R800/R550) − 1 | [48,49] |
| Red edge chlorophyll index (CIred edge) | (R800/R720) − 1 | [48,49] |
| Vegetation Index | Calibration | Validation | Reference | |||
|---|---|---|---|---|---|---|
| Equation | R2 | R2 | RMSE | RRMSE | ||
| EVI | y = 6.74x − 0.67 | 0.25 | 0.23 | 0.49 | [45] | |
| NDVI | y = 8.67x – 2.62 | 0.35 | 0.33 | 0.45 | 14.9% | [44] |
| Green NDVI | y = 0.91e2.39x | 0.64 | 0.61 | 0.38 | 11.30% | [47] |
| REP | y = 0.18x − 125.03 | 0.65 | 0.63 | 0.33 | 10.991% | [46] |
| CIgreen | y = 1.62e0.30x | 0.67 | 0.63 | 0.36 | 11.10% | [48,49] |
| CIred edge | y = 1.70e1.30x | 0.71 | 0.68 | 0.30 | 10.54% | [48,49] |
| ↓FY687/↓FY739 | y = −ln(x) + 1.56 | 0.75 | 0.74 | 0.28 | 9.25% | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Zhu, J.; Ma, C.; Alonso, L.; Li, D.; Cheng, T.; Tian, Y.; Zhu, Y.; Yao, X.; Cao, W. Difference and Potential of the Upward and Downward Sun-Induced Chlorophyll Fluorescence on Detecting Leaf Nitrogen Concentration in Wheat. Remote Sens. 2018, 10, 1315. https://doi.org/10.3390/rs10081315
Jia M, Zhu J, Ma C, Alonso L, Li D, Cheng T, Tian Y, Zhu Y, Yao X, Cao W. Difference and Potential of the Upward and Downward Sun-Induced Chlorophyll Fluorescence on Detecting Leaf Nitrogen Concentration in Wheat. Remote Sensing. 2018; 10(8):1315. https://doi.org/10.3390/rs10081315
Chicago/Turabian StyleJia, Min, Jie Zhu, Chunchen Ma, Luis Alonso, Dong Li, Tao Cheng, Yongchao Tian, Yan Zhu, Xia Yao, and Weixing Cao. 2018. "Difference and Potential of the Upward and Downward Sun-Induced Chlorophyll Fluorescence on Detecting Leaf Nitrogen Concentration in Wheat" Remote Sensing 10, no. 8: 1315. https://doi.org/10.3390/rs10081315
APA StyleJia, M., Zhu, J., Ma, C., Alonso, L., Li, D., Cheng, T., Tian, Y., Zhu, Y., Yao, X., & Cao, W. (2018). Difference and Potential of the Upward and Downward Sun-Induced Chlorophyll Fluorescence on Detecting Leaf Nitrogen Concentration in Wheat. Remote Sensing, 10(8), 1315. https://doi.org/10.3390/rs10081315