Using Hyperspectral Crop Residue Angle Index to Estimate Maize and Winter-Wheat Residue Cover: A Laboratory Study
Abstract
:1. Introduction
2. Background and Proposed Crop Residue Angle Index
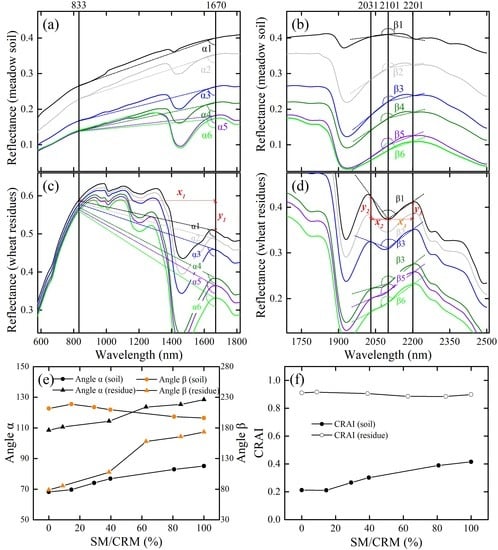
2.1. Response to Moisture of Crop Residue and Soil Reflectance
2.2. Proposed Crop Residue Angle Index and Its Response to Moisture
2.3. Traditional Crop Residue Cover Spectral Indices
3. Laboratory Data Collection
3.1. Laboratory Measurements
3.1.1. Hyperspectral Measurements
3.1.2. Crop Residue Cover and Moisture Measurements
3.2. Estimate of Crop Residue Cover and Statistical Analysis
3.2.1. Estimate of Crop Residue Cover
3.2.2. Statistical Analysis
4. Results
4.1. Selection of Traditional Broad-Band Spectral Indices
4.2. Response of Spectral Indices to Moisture and Soil Background
4.2.1. Response of Spectral Indices to Moisture
4.2.2. Response of Spectral Indices to Soil Background
4.3. Estimation of Crop Residue Cover
5. Analysis and Discussion
5.1. Analysis of Spectral Indices for Crop Residue Cover Using Laboratory Dataset
5.2. Limitations and Future Application of Laboratory-Based CRAI
6. Conclusions
- (i)
- Mitigating the uncertain effect caused by moisture is of significant importance to properly estimate CRC using remote-sensing techniques. Crop residue moisture content significantly affects the traditional SIs (Table 3, Figure 6) except for SINDRI. All broad-band indices are less correlated with CRC when using all datasets than when using only the dry, wet, or saturated dataset (Table 3). Although the CAI provides the best estimate of CRC (r = 0.869) when using the dry dataset, it leads to a poor estimate of CRC (r = 0.580) when crop residue samples have varying moisture content (Table 3).
- (ii)
- In this work, the proposed CRAI accurately estimates the CRC regardless of soil, soil moisture, or crop residue moisture by using a laboratory-based dataset (Table 2). The CRAI combines two features that reflect the moisture content in soil and crop residue. The first is the different reflectance of soil and crop residue as a function of moisture in the near-infrared band (833 nm) and short-wave near-infrared band (1670 nm), and the second is different reflectance of soils and crop residues to lignin, cellulose, and moisture in the bands at 2101, 2031, and 2201 nm. However, note that the CRAI has yet to be verified in the field. Thus, above-mentioned advantages of new proposed index require additional real satellite-based hyperspectral remote sensing testing.
- (iii)
- The current study uses laboratory-based tests, which allows us to compare samples with different moisture content ((i) dry, (ii) wet, (iii) saturated, and (iv) all datasets). To confirm that the findings apply to a broader range of crops and ecological areas, additional field-based experiments are planned.
- (iv)
- Because numerous broadband remote-sensing data are free and available, a multispectral crop residue angle index will be more valuable than the hyperspectral version presented herein. Therefore, broad-band multi-spectral reflectance respond to SM/CRM should be analyzed in future works.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Soil Name | City | Color | Soil Order | Clay a | Silt b | Sand c | Soil Type | PH |
|---|---|---|---|---|---|---|---|---|
| Paddy Soil | Nanjing | 10YR4/3 | Anthrosols | 99.95% | 0.05% | 0.00% | Clay | 6.46 |
| Black Soil | Jining | 10YR4/3 | Vertisols | 87.00% | 6.83% | 6.17% | Clay | 7.36 |
| Meadow Soil | Jining | 2.5YR7/4 | Histosols | 76.7% | 20.4% | 2.90% | Clay | 7.74 |
| Brown Soil | Jining | 2.5YR6/3 | Alfisols | 71.10% | 11.70% | 17.20% | Clay | 6.54 |
| Types | Indices | Saturated | Wet | Dry | All |
|---|---|---|---|---|---|
| Wheat | CRAI | 0.981 | 0.947 | 0.943 | 0.932 |
| CAI | 0.920 | 0.850 | 0.919 | 0.628 | |
| SINDRI | 0.914 | 0.922 | 0.936 | 0.910 | |
| NDSVI | 0.893 | 0.764 | 0.839 | 0.665 | |
| SGNDI | 0.913 | 0.823 | 0.930 | 0.692 | |
| SRNDI | 0.911 | 0.816 | 0.941 | 0.666 | |
| Maize | CRAI | 0.922 | 0.911 | 0.927 | 0.859 |
| CAI | 0.817 | 0.678 | 0.892 | 0.502 | |
| SINDRI | 0.836 | 0.868 | 0.804 | 0.793 | |
| NDSVI | 0.857 | 0.797 | 0.754 | 0.657 | |
| SGNDI | 0.776 | 0.750 | 0.788 | 0.576 | |
| SRNDI | 0.761 | 0.737 | 0.763 | 0.552 |
| Types | Indices | Paddy Soil | Black Soil | Meadow Soil | Brown Soil | Four Soils |
|---|---|---|---|---|---|---|
| Dry | CRAI | 0.979 | 0.992 | 0.989 | 0.957 | 0.943 |
| CAI | 0.971 | 0.973 | 0.970 | 0.957 | 0.919 | |
| SINDRI | 0.958 | 0.968 | 0.975 | 0.953 | 0.936 | |
| NDSVI | 0.877 | 0.976 | 0.953 | 0.838 | 0.839 | |
| SGNDI | 0.953 | 0.986 | 0.984 | 0.943 | 0.930 | |
| SRNDI | 0.959 | 0.986 | 0.985 | 0.932 | 0.941 | |
| Saturated | CRAI | 0.995 | 0.980 | 0.995 | 0.996 | 0.981 |
| CAI | 0.965 | 0.852 | 0.965 | 0.977 | 0.920 | |
| SINDRI | 0.928 | 0.840 | 0.928 | 0.978 | 0.914 | |
| NDSVI | 0.980 | 0.920 | 0.980 | 0.937 | 0.893 | |
| SGNDI | 0.978 | 0.868 | 0.978 | 0.940 | 0.913 | |
| SRNDI | 0.967 | 0.927 | 0.967 | 0.948 | 0.911 |
| Crops | Indices | Calibration (279 Wheat and 261 Maize) | Validation (556 Wheat and 520 Maize) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regression Model | MAE | RMSE | nRMSE | R2 | MAE | RMSE | nRMSE | R2 | ||
| Wheat | CRAI | y = 159.52 x − 43.67 | 7.99 | 9.64 | 10.28 | 0.870 | 7.80 | 9.54 | 10.46 | 0.872 |
| CAI | y = 11.98 x + 43.14 | 16.30 | 19.80 | 21.11 | 0.451 | 17.13 | 20.49 | 22.47 | 0.411 | |
| SINDRI (l) | y = 12.74 x + 4.54 | 9.15 | 11.22 | 11.97 | 0.824 | 8.95 | 11.03 | 12.10 | 0.830 | |
| SINDRI (e) | y = 11.57 exp (0.35 x) | 8.59 | 10.86 | 11.50 | 0.842 | 8.53 | 10.64 | 11.10 | 0.846 | |
| NDSVI | y = −223.99 x + 81.10 | 17.02 | 20.43 | 21.78 | 0.416 | 15.85 | 19.56 | 21.45 | 0.462 | |
| SGNDI | y = 152.47 x + 72.55 | 16.20 | 19.70 | 21.00 | 0.457 | 15.19 | 18.87 | 20.69 | 0.500 | |
| SRNDI | y = −131.65 x + 48.31 | 16.81 | 20.32 | 21.66 | 0.422 | 15.57 | 19.35 | 21.22 | 0.474 | |
| Maize | CRAI | y = 271.1 x - 78.63 | 11.21 | 13.48 | 13.93 | 0.738 | 11.14 | 13.90 | 14.85 | 0.730 |
| CAI | y = 10.53 x + 49.65 | 19.10 | 22.96 | 23.72 | 0.252 | 19.36 | 22.93 | 24.49 | 0.260 | |
| SINDRI (l) | y = 21.60 x − 3.69 | 13.09 | 16.13 | 16.66 | 0.629 | 12.99 | 15.73 | 16.81 | 0.654 | |
| SINDRI (e) | y = 11.73 exp (0.51 x) | 14.79 | 20.38 | 21.10 | 0.498 | 14.43 | 19.97 | 21.30 | 0.526 | |
| NDSVI | y = −208.59 x + 78.12 | 16.75 | 19.92 | 20.58 | 0.432 | 16.43 | 19.65 | 21.00 | 0.461 | |
| SGNDI | y = 112.82 x + 65.70 | 18.26 | 21.65 | 22.37 | 0.331 | 17.96 | 21.27 | 22.73 | 0.368 | |
| SRNDI | y = −115.69 x + 53.40 | 18.61 | 22.08 | 22.81 | 0.305 | 18.36 | 21.71 | 23.20 | 0.341 | |
References
- Mu, X.; Zhao, Y.; Liu, K.; Ji, B.; Guo, H.; Xue, Z.; Li, C. Responses of soil properties, root growth and crop yield to tillage and crop residue management in a wheat-maize cropping system on the North China Plain. Eur. J. Agron. 2016, 78, 32–43. [Google Scholar] [CrossRef]
- Kimble, J.M.; Follett, R.F.; Cole, C.V. The Potential of US Cropland to Sequester Carbon and Mitigate the Greenhouse Effect; CRC Press: Boca Raton, FL, USA, 1998; ISBN 157504112X. [Google Scholar]
- Erenstein, O. Crop residue mulching in tropical and semi-tropical countries: An evaluation of residue availability and other technological implications. Soil Tillage Res. 2002, 67, 115–133. [Google Scholar] [CrossRef]
- Lal, R.; Bruce, J.P. The potential of world cropland soils to sequester C and mitigate the greenhouse effect. Environ. Sci. Policy 1999. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Serbin, G.; Reeves, J.B.; Doraiswamy, P.C.; Hunt, E.R. Spectral reflectance of wheat residue during decomposition and remotely sensed estimates of residue cover. Remote Sens. 2010, 2, 416–431. [Google Scholar] [CrossRef]
- Zhang, T.; Wooster, M.J.; Xu, W. Remote Sensing of Environment Approaches for synergistically exploiting VIIRS I- and M-Band data in regional active fi re detection and FRP assessment: A demonstration with respect to agricultural residue burning in Eastern China. Remote Sens. Environ. 2017, 198, 407–424. [Google Scholar] [CrossRef]
- Chen, S.; Xu, C.; Yan, J.; Zhang, X.; Zhang, X.; Wang, D. The influence of the type of crop residue on soil organic carbon fractions: An 11-year field study of rice-based cropping systems in southeast China. Agric. Ecosyst. Environ. 2016, 223, 261–269. [Google Scholar] [CrossRef]
- Eriksen-Hamel, N.S.; Speratti, A.B.; Whalen, J.K.; Légère, A.; Madramootoo, C.A. Earthworm populations and growth rates related to long-term crop residue and tillage management. Soil Tillage Res. 2009, 104, 311–316. [Google Scholar] [CrossRef]
- Morrison, J.E.; Lemunyon, J.; Bogusch, H.C. Sources of variation and performance of 9 devices when measuring percent residue cover. Trans. ASAE 1995, 38, 521–529. [Google Scholar] [CrossRef]
- Morrison, J.E.; Huang, C.-H.; Lightle, D.T.; Daughty, C.S.T. Residue measurement techniques. J. Soil Water Conserv. 1993, 48, 478–483. [Google Scholar]
- Li, Z.; Guo, X. Non-photosynthetic vegetation biomass estimation in semiarid Canadian mixed grasslands using ground hyperspectral data, Landsat 8 OLI, and Sentinel-2 images. Int. J. Remote Sens. 2018, 39, 6893–6913. [Google Scholar] [CrossRef]
- Hamidisepehr, A.; Sama, M.P.; Turner, A.P.; Wendroth, O.O. A Method for Reflectance Index Wavelength Selection from Moisture-Controlled Soil and Crop Residue Samples. Trans. ASABE 2017, 60, 1479–1487. [Google Scholar] [CrossRef]
- Hill, M.J.; Román, M.O.; Schaaf, C.B. Dynamics of vegetation indices in tropical and subtropical savannas defined by ecoregions and Moderate Resolution Imaging Spectroradiometer (MODIS) land cover. Geocarto Int. 2012, 27, 153–191. [Google Scholar] [CrossRef]
- Hively, W.D.; Lamb, B.T.; Daughtry, C.S.T.; Shermeyer, J.; McCarty, G.W.; Quemada, M. Mapping crop residue and tillage intensity using WorldView-3 satellite shortwave infrared residue indices. Remote Sens. 2018, 10, 1657. [Google Scholar] [CrossRef]
- Ridd, M.K. Exploring a V-I-S (Vegetation-impervious surface-soil) model for urban ecosystem analysis through remote sensing: Comparative anatomy for citiest. Int. J. Remote Sens. 1995, 16, 2165–2185. [Google Scholar] [CrossRef]
- Wu, M.; Wang, J.; Wang, G.; Zou, X.; Wang, Z.; Chai, G. Estimating the fractional cover of photosynthetic vegetation, non-photosynthetic vegetation and bare soil from MODIS data: Assessing the applicability of the NDVI-DFI model in the typical Xilingol grasslands. Int. J. Appl. Earth Obs. Geoinf. 2019, 76, 154–166. [Google Scholar] [CrossRef]
- Sonmez, N.K.; Slater, B. Measuring intensity of tillage and plant residue cover using remote sensing. Eur. J. Remote Sens. 2016, 49, 121–135. [Google Scholar] [CrossRef]
- Wang, C.K.; Pan, X.Z.; Liu, Y.; Li, Y.L.; Shi, R.J.; Zhou, R.; Xie, X.L. Alleviating moisture effects on remote sensing estimation of crop residue cover. Agron. J. 2013, 105, 967–976. [Google Scholar] [CrossRef]
- Daughtry, C.S.; Beeson, P.C.; Milak, S.; Akhmedov, B.; Sadeghi, A.M.; Hunt, E.R.; Tomer, M.D. Assessing the extent of conservation tillage in agricultural landscapes. In Proceedings of the Remote Sensing for Agriculture, Ecosystems, and Hydrology XIV, Edinburgh, Scotland, 24–26 September 2012; Volume 8531. [Google Scholar] [CrossRef]
- Daughtry, C.S.T. Discriminating crop residues from soil by shortwave infrared reflectance. Agron. J. 2001, 93, 125–131. [Google Scholar] [CrossRef]
- Wang, C. Estimation of Crop Residue Cover by Remote Rensing and Correction of Moisture Effects on Estimates; University of Chinese Academy of Sciences: Beijing, China, 2013. [Google Scholar]
- Daughtry, C.S.T.; Hunt, E.R. Mitigating the effects of soil and residue water contents on remotely sensed estimates of crop residue cover. Remote Sens. Environ. 2008, 112, 1647–1657. [Google Scholar] [CrossRef]
- Wang, C.; Pan, X.; Liu, Y.; Li, Y.; Zhou, R.; Xie, X. Modeling the Effect of Moisture on the Reflectance of Crop Residues. Agron. J. 2012, 104, 1652–1657. [Google Scholar] [CrossRef]
- Quemada, M.; Daughtry, C.S.T. Spectral indices to improve crop residue cover estimation under varying moisture conditions. Remote Sens. 2016, 8, 660. [Google Scholar] [CrossRef]
- Serbin, G.; Daughtry, C.S.T.; Hunt, E.R.; Brown, D.J.; McCarty, G.W. Effect of Soil Spectral Properties on Remote Sensing of Crop Residue Cover. Soil Sci. Soc. Am. J. 2009, 73, 1545–1558. [Google Scholar] [CrossRef]
- Serbin, G.; Daughtry, C.S.T.; Hunt, E.R.; Reeves, J.B.; Brown, D.J. Remote Sensing of Environment Effects of soil composition and mineralogy on remote sensing of crop residue cover. Remote Sens. Environ. 2009, 113, 224–238. [Google Scholar] [CrossRef]
- Omar, Z.; Stathaki, T. Hyperspectral unmixing overview: Geometrical, statistical, and sparse regression-based approaches. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 354–379. [Google Scholar] [CrossRef]
- Bateson, A.; Curtis, B. A method for manual endmembers selection and spectral unmixing. Remote Sens. Environ. 1996, 55, 229–243. [Google Scholar] [CrossRef]
- Pacheco, A.; McNairn, H. Evaluating multispectral remote sensing and spectral unmixing analysis for crop residue mapping. Remote Sens. Environ. 2010, 114, 2219–2228. [Google Scholar] [CrossRef]
- Cao, X.; Chen, J.; Matsushita, B.; Imura, H. Developing a MODIS-based index to discriminate dead fuel from photosynthetic vegetation and soil background in the Asian steppe area. Int. J. Remote Sens. 2010, 31, 1589–1604. [Google Scholar] [CrossRef]
- Mcnairn, H.; Protz, R. Mapping corn residue cover on agricultural fields in oxford county, ontario, using thematic mapper. Can. J. Remote Sens. 1993, 19, 152–159. [Google Scholar] [CrossRef]
- Van Deventer, A.P.; Ward, A.D.; Gowda, P.M.H.M.; Lyon, J.G. Using thematic mapper data to identify contrasting soil plains and tillage practices. Photogramm. Eng. Remote Sens. 1997, 63, 87–93. [Google Scholar] [CrossRef]
- Qi, J.; Marsett, R.; Heilman, P.; Bieden-bender, S.; Moran, S.; Goodrich, D.; Weltz, M. Ranges improves satellite-based information and land cover assessments in southwest United States. Eos Trans. Am. Geophys. Union 2002, 83. [Google Scholar] [CrossRef]
- Jin, X.; Ma, J.; Wen, Z.; Song, K. Estimation of maize residue cover using Landsat-8 OLI image spectral information and textural features. Remote Sens. 2015, 7, 14559–14575. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; McMurtrey, J.E.; Chappelle, E.W.; Hunter, W.J.; Steiner, J.L. Measuring crop residue cover using remote sensing techniques. Theor. Appl. Climatol. 1996, 54, 17–26. [Google Scholar] [CrossRef]
- Serbin, G.; Hunt, E.R.; Daughtry, C.S.T.; McCarty, G.W.; Doraiswamy, P.C. An improved ASTER index for remote sensing of crop residue. Remote Sens. 2009, 1, 971–991. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E. Remote sensing the spatial distribution of crop residues. Agron. J. 2005, 97, 864–871. [Google Scholar] [CrossRef]
- Biard, F.; Baret, F. Crop residue estimation using multiband reflectance. Remote Sens. Environ. 1997, 59, 530–536. [Google Scholar] [CrossRef]
- Guerschman, J.P.; Hill, M.J.; Renzullo, L.J.; Barrett, D.J.; Marks, A.S.; Botha, E.J. Estimating fractional cover of photosynthetic vegetation, non-photosynthetic vegetation and bare soil in the Australian tropical savanna region upscaling the EO-1 Hyperion and MODIS sensors. Remote Sens. Environ. 2009, 113, 928–945. [Google Scholar] [CrossRef]
- Quemada, M.; Hively, W.D.; Daughtry, C.S.T.; Lamb, B.T.; Shermeyer, J. Improved crop residue cover estimates obtained by coupling spectral indices for residue and moisture. Remote Sens. Environ. 2018, 206, 33–44. [Google Scholar] [CrossRef]
- Yue, J.; Tian, Q.; Tang, S.; Xu, K.; Zhou, C. A dynamic soil endmember spectrum selection approach for soil and crop residue linear spectral unmixing analysis. Int. J. Appl. Earth Obs. Geoinf. 2019, 78, 306–317. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F.; Hanocq, J.F. Modeling spectral and bidirectional soil reflectance. Remote Sens. Environ. 1992, 41, 123–132. [Google Scholar] [CrossRef]
- Galvão, L.S.; Vitorello, Í. Variability of laboratory measured soil lines of soils from southeastern Brazil. Remote Sens. Environ. 1998, 63, 166–181. [Google Scholar] [CrossRef]
- Muller, E.; Décamps, H. Modeling soil moisture–reflectance. Remote Sens. Environ. 2001, 76, 173–180. [Google Scholar] [CrossRef]
- Twomey, S.A.; Bohren, C.F.; Merganthaler, J.L. Reflectance and albedo difference between wet and dry surfaces. Appl. Opt. 1986, 25, 431–437. [Google Scholar] [CrossRef]
- Palmer, K.F.; Williams, D. Optical properties of water in the near infrared. J. Opt. Soc. Am. 1974, 64, 1107–1110. [Google Scholar] [CrossRef]
- Nagler, P.L.; Daughtry, C.S.T.; Goward, S.N. Plant litter and soil reflectance. Remote Sens. Environ. 2000, 71, 207–215. [Google Scholar] [CrossRef]
- Sullivan, D.G.; Truman, C.C.; Schomberg, H.H.; Endale, D.M.; Strickland, T.C. Evaluating techniques for determining tillage regime in the Southeastern Coastal Plain and piedmont. Agron. J. 2006, 98, 1236–1246. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Hunt, E.R.; McMurtrey, J.E. Assessing crop residue cover using shortwave infrared reflectance. Remote Sens. Environ. 2004, 90, 126–134. [Google Scholar] [CrossRef]
- Nagler, P.L.; Inoue, Y.; Glenn, E.P.; Russ, A.L.; Daughtry, C.S.T. Cellulose absorption index (CAI) to quantify mixed soil-plant litter scenes. Remote Sens. Environ. 2003, 87, 310–325. [Google Scholar] [CrossRef]
- Dashti, H.; Glenn, N.F.; Spaete, L.P.; Ilangakoon, N. Hyperspectral Imagery from AVIRIS-NG for Sites in ID and CA, USA, 2014 and 2015; ORNL DAAC: Oak Ridge, TN, USA, 2018. [Google Scholar] [CrossRef]
- Bannari, A.; Staenz, K.; Champagne, C.; Khurshid, K.S. Spatial variability mapping of crop residue using hyperion (EO-1) hyperspectral data. Remote Sens. 2015, 7, 8107–8127. [Google Scholar] [CrossRef]
- ESA Compact High Resolution Imaging Spectrometer. Available online: https://earth.esa.int/web/guest/missions/esa-operational-eo-missions/proba (accessed on 25 March 2019).
- Sun, Y.; Jiang, G.; Yunduan, L.I.; Yang, Y.; Dai, H.; Jun, H.E.; Qinghao, Y.E.; Cao, Q.; Dong, C.; Zhao, S. GF-5 Satellite: Overview and Application Prospects. Spacecr. Recover. Remote Sens. 2018, 39, 1–13. [Google Scholar] [CrossRef]











| Type | Spectral Indices | Equation | Reference |
|---|---|---|---|
| Broad-band | SGNDI | (OLI3 - OLI7)/ (OLI3 + OLI7), | This paper |
| STI | OLI6/OLI7, | [32] | |
| MCRC | (OLI6 - OLI3)/ (OLI6 + OLI3), | [48] | |
| SRNDI | (OLI7 - OLI4)/ (OLI7 + OLI4), | [34] | |
| DFI | 100 × (1 - OLI7/OLI6)/ (OLI4/OLI5), | [30] | |
| NDI5 | (OLI5 - OLI6)/ (OLI5 + OLI6), | [31] | |
| NDI7 | (OLI5 - OLI7)/ (OLI5 + OLI7), | [31] | |
| NDTI | (OLI6 - OLI7)/ (OLI6 + OLI7), | [32] | |
| NDSVI | (OLI6 - OLI4)/ (OLI6 + OLI4), | [33] | |
| Hyperspectral | SINDRI | 100 × (R2210 − R2260)/(R2210 + R2260) 100 × (A6 − A7)/(A6 + A7), | [36] |
| CAI | 100 × ((R2031+R2201)/2-R2101), | [35] |
| Soils | Groups | Maize | Wheat | |||||
|---|---|---|---|---|---|---|---|---|
| Samples | SM | CRM | Samples | SM | CRM | |||
| Brown soil | Dry | 44 | 0 | 0 | 43 | 0 | 0 | |
| Wet | Wet2 | 48 | 12.1 | 20.0 | 54 | 12.1 | 50.0 | |
| Wet1 | 52 | 34.3 | 35.0 | 54 | 34.3 | 86.1 | ||
| Saturated | 46 | 100 | 100 | 46 | 100 | 100 | ||
| Black Soil | Dry | 53 | 0 | 0 | 53 | 0 | 0 | |
| Wet | Wet2 | 53 | 20.3 | 18.5 | 56 | 20.3 | 13.7 | |
| Wet1 | 48 | 66.6 | 52.2 | 54 | 66.6 | 40.2 | ||
| Saturated | 52 | 100 | 100 | 58 | 100 | 100 | ||
| Meadow Soil | Dry | 52 | 0 | 0 | 53 | 0 | 0 | |
| Wet | Wet2 | 53 | 17.9 | 18.5 | 50 | 17.9 | 13.7 | |
| Wet1 | 46 | 45.5 | 52.2 | 53 | 45.5 | 40.2 | ||
| Saturated | 46 | 100 | 100 | 55 | 100 | 100 | ||
| Paddy Soil | Dry | 46 | 0 | 0 | 48 | 0 | 0 | |
| Wet | Wet2 | 47 | 13.2 | 20.0 | 55 | 13.2 | 50.0 | |
| Wet1 | 49 | 54.3 | 35.0 | 50 | 54.3 | 86.1 | ||
| Saturated | 46 | 100 | 100 | 53 | 100 | 100 | ||
| Total | 781 | 835 | ||||||
| Ranking | Samples and Dataset | |||||||
|---|---|---|---|---|---|---|---|---|
| Dry (n = 392) | Wet (n = 822) | Saturated (n = 402) | All (n = 1616) | |||||
| Indices | |r| | Indices | |r| | Indices | |r| | Indices | |r| | |
| 1 | SGNDI | 0.842** | SGNDI | 0.787** | NDSVI | 0.856** | NDSVI | 0.685** |
| 2 | SRNDI | 0.833** | SRNDI | 0.787** | SRNDI | 0.827** | MCRC | 0.658** |
| 3 | DFI | 0.775** | NDSVI | 0.786** | SGNDI | 0.81** | SGNDI | 0.653** |
| 4 | NDSVI | 0.768** | MCRC | 0.704** | MCRC | 0.741** | SRNDI | 0.629** |
| 5 | NDI7 | 0.744** | NDI5 | 0.677** | NDI5 | 0.698** | NDI5 | 0.587** |
| 6 | MCRC | 0.733** | DFI | 0.656** | NDI7 | 0.671** | NDI7 | 0.546** |
| 7 | NDI5 | 0.726** | NDI7 | 0.656** | DFI | 0.552** | DFI | 0.498** |
| 8 | NDTI | 0.716** | NDTI | 0.558** | STI | 0.498** | NDTI | 0.444** |
| 9 | STI | 0.709** | STI | 0.557** | NDTI | 0.495** | STI | 0.434** |
| - | SINDRI | 0.815** | SINDRI | 0.790** | SINDRI | 0.737** | SINDRI | 0.776** |
| - | CAI | 0.869** | CAI | 0.780** | CAI | 0.777** | CAI | 0.580** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, J.; Tian, Q.; Dong, X.; Xu, K.; Zhou, C. Using Hyperspectral Crop Residue Angle Index to Estimate Maize and Winter-Wheat Residue Cover: A Laboratory Study. Remote Sens. 2019, 11, 807. https://doi.org/10.3390/rs11070807
Yue J, Tian Q, Dong X, Xu K, Zhou C. Using Hyperspectral Crop Residue Angle Index to Estimate Maize and Winter-Wheat Residue Cover: A Laboratory Study. Remote Sensing. 2019; 11(7):807. https://doi.org/10.3390/rs11070807
Chicago/Turabian StyleYue, Jibo, Qingjiu Tian, Xinyu Dong, Kaijian Xu, and Chengquan Zhou. 2019. "Using Hyperspectral Crop Residue Angle Index to Estimate Maize and Winter-Wheat Residue Cover: A Laboratory Study" Remote Sensing 11, no. 7: 807. https://doi.org/10.3390/rs11070807
APA StyleYue, J., Tian, Q., Dong, X., Xu, K., & Zhou, C. (2019). Using Hyperspectral Crop Residue Angle Index to Estimate Maize and Winter-Wheat Residue Cover: A Laboratory Study. Remote Sensing, 11(7), 807. https://doi.org/10.3390/rs11070807