Spatial Habitat Shifts of Oceanic Cephalopod (Ommastrephes bartramii) in Oscillating Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pacific Decadal Oscillation (PDO)-Based Climatic Phase Shifts
2.2. Analytical Framework
2.3. Squid Occurrences and Environmental Data
2.4. Multi-Model Ensemble of Potential Squid Habitat
2.5. Computing for Temporal Trends at Each Climatic Transition
3. Results
3.1. Squid Environmental Preferences
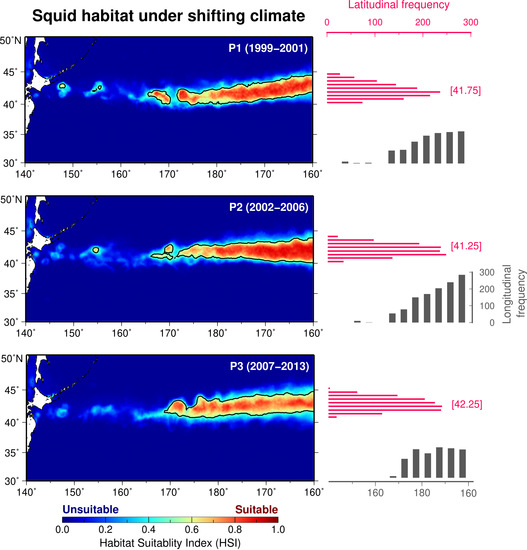
3.2. Spatial Patterns of Period-Specific Squid Habitat Forecasts
3.3. Temporal Trends in Squid Habitat and Environmental Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roper, C.F.E.; Sweeney, M.; Nauen, C. Cephalopods of the World, an Annotated and illustrated Catalogue of Species of Interest to Fisheries; FAO Fish Finder: Rome, Italy, 1984; Volume 3, pp. 1–277. [Google Scholar]
- Ichii, T.; Mahapatra, K.; Okamura, H.; Okada, Y. Stock assessment of the autumn cohort of neon flying squid (Ommastrephes bartramii) in the north pacific based on past large-scale high seas driftnet fishery data. Fish. Res. 2006, 78, 286–297. [Google Scholar] [CrossRef]
- Chen, X.; Liu, B.; Chen, Y. A review of the development of Chinese distant-water squid jigging fisheries. Fish. Res. 2008, 89, 211–221. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Rodhouse, P.G.K.; Pierce, G.J.; Sauer, W.; Sakai, M.; Allcock, L.; Arguelles, J.; Bower, J.R.; Castillo, G.; Ceriola, L.; et al. World squid fisheries. Rev. Fish. Sci. Aquac. 2015, 23, 92–252. [Google Scholar] [CrossRef] [Green Version]
- Yatsu, A.; Midorikawa, S.; Shimada, T.; Uozumi, Y. Age and growth of the neon flying squid, Ommastrephes bartrami, in the North Pacific Ocean. Fish. Res. 1997, 29, 257–270. [Google Scholar] [CrossRef]
- Alabia, I.D.; Saitoh, S.-I.; Hirawake, T.; Igarashi, H.; Ishikawa, Y.; Usui, N.; Kamachi, M.; Awaji, T.; Seito, M. Elucidating the potential squid habitat responses in the central North Pacific to the recent ENSO flavors. Hydrobiologia 2016, 772, 1–13. [Google Scholar] [CrossRef]
- Ichii, T.; Mahapatra, K.; Sakai, M.; Wakabayashi, T.; Okamura, H.; Igarashi, H.; Inagake, D.; Okada, Y. Changes in abundance of the neon flying squid Ommastrephes bartramii in relation to climate change in the central North Pacific Ocean. Mar. Ecol. Prog. Ser. 2011, 441, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, H.; Ichii, T.; Sakai, M.; Ishikawa, Y.; Toyoda, T.; Masuda, S.; Sugiura, N.; Mahapatra, K.; Awaji, T. Possible link between interannual variation of neon flying squid (Ommastrephes bartramii) abundance in the North Pacific and the climate phase shift in 1998/1999. Prog. Oceanogr. 2017, 150, 20–34. [Google Scholar] [CrossRef]
- Ichii, T.; Mahapatra, K.; Sakai, M.; Okada, Y. Life history of the neon flying squid: Effect of the oceanographic regime in the North Pacific Ocean. Mar. Ecol. Prog. Ser. 2009, 378, 1–11. [Google Scholar] [CrossRef]
- Doubleday, Z.A.; Prowse, T.A.A.; Arkhipkin, A.; Pierce, G.J.; Semmens, J.; Steer, M.; Leporati, S.C.; Lourenço, S.; Quetglas, A.; Sauer, W.; et al. Global proliferation of cephalopods. Curr. Biol. 2016, 26, R406–R407. [Google Scholar] [CrossRef] [Green Version]
- Bower, J.R.; Ichii, T. The red flying squid (Ommastrephes bartramii): A review of recent research and the fishery in Japan. Fish. Res. 2005, 76, 39–55. [Google Scholar] [CrossRef]
- Parry, M. Feeding behavior of two ommastrephid squids Ommastrephes bartramii and Sthenoteuthis oualaniensis off Hawaii. Mar. Ecol. Prog. Ser. 2006, 318, 229–235. [Google Scholar] [CrossRef]
- Watanabe, H.; Kubodera, T.; Ichii, T.; Kawahara, S. Feeding habits of neon flying squid Ommastrephes bartramii in the transitional region of the central North Pacific. Mar. Ecol. Prog. Ser. 2004, 266, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Bates, A.E.; Pecl, G.T.; Frusher, S.; Hobday, A.J.; Wernberg, T.; Smale, D.A.; Sunday, J.M.; Hill, N.A.; Dulvy, N.K.; Colwell, R.K.; et al. Defining and observing stages of climate-mediated range shifts in marine systems. Glob. Environ. Chang. 2014, 26, 27–38. [Google Scholar] [CrossRef]
- Gunderson, A.R.; Armstrong, E.J.; Stillman, J.H. Multiple stressors in a changing world: The need for an improved perspective on physiological responses to the dynamic marine environment. Annu. Rev. Mar. Sci. 2016, 8, 357–378. [Google Scholar] [CrossRef]
- Salinger, M.J.; Bell, J.D.; Evans, K.; Hobday, A.J.; Allain, V.; Brander, K.; Dexter, P.; Harrison, D.E.; Hollowed, A.B.; Lee, B.; et al. Climate and oceanic fisheries: Recent observations and projections and future needs. Clim. Chang. 2013, 119, 213–221. [Google Scholar] [CrossRef]
- Anderson, C.I.H.; Rodhouse, P.G. Life cycles, oceanography and variability: Ommastrephid squid in variable oceanographic environments. Fish. Res. 2001, 54, 133–143. [Google Scholar] [CrossRef]
- Rodhouse, P.G. Managing and forecasting squid fisheries in variable environments. Fish. Res. 2001, 54, 3–8. [Google Scholar] [CrossRef]
- Abrahms, B.; Welch, H.; Brodie, S.; Jacox, M.G.; Becker, E.A.; Bograd, S.J.; Irvine, L.M.; Palacios, D.M.; Mate, B.R.; Hazen, E.L. Dynamic ensemble models to predict distributions and anthropogenic risk exposure for highly mobile species. Divers. Distrib. 2019, 25, 1182–1193. [Google Scholar] [CrossRef] [Green Version]
- Burgeot, T.; Akcha, F.; Ménard, D.; Robinson, C.; Loizeau, V.; Brach-Papa, C.; Martínez-Gòmez, C.; Le Goff, J.; Budzinski, H.; Le Menach, K.; et al. Integrated monitoring of chemicals and their effects on four sentinel species, Limanda limanda, Platichthys flesus, Nucella lapillus and Mytilus sp., in Seine Bay: A key step towards applying biological effects to monitoring. Mar. Environ. Res. 2017, 124, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Fossi, M.C.; Panti, C.; Baini, M.; Lavers, J.L. A review of plastic-associated pressures: Cetaceans of the mediterranean sea and eastern australian shearwaters as case studies. Front. Mar. Sci. 2018, 5, 173. [Google Scholar] [CrossRef]
- Alabia, I.D.; Saitoh, S.-I.; Mugo, R.; Igarashi, H.; Ishikawa, Y.; Usui, N.; Kamachi, M.; Awaji, T.; Seito, M. Seasonal potential fishing ground prediction of neon flying squid (Ommastrephes bartramii) in the western and central North Pacific. Fish. Oceanogr. 2015, 24, 190–203. [Google Scholar] [CrossRef]
- Alabia, I.D.; Saitoh, S.-I.; Mugo, R.; Igarashi, H.; Ishikawa, Y.; Usui, N.; Kamachi, M.; Awaji, T.; Seito, M. Identifying pelagic habitat hotspots of neon flying squid in the temperate waters of the central North Pacific. PLoS ONE 2015, 10, e0142885. [Google Scholar] [CrossRef] [Green Version]
- Keppel, G.; Van Niel, K.P.; Wardell-Johnson, G.W.; Yates, C.J.; Byrne, M.; Mucina, L.; Schut, A.G.T.; Hopper, S.D.; Franklin, S.E. Refugia: Identifying and understanding safe havens for biodiversity under climate change. Glob. Ecol. Biogeogr. 2012, 21, 393–404. [Google Scholar] [CrossRef]
- Ashcroft, M.B. Identifying refugia from climate change. J. Biogeogr. 2010, 37, 1407–1413. [Google Scholar] [CrossRef]
- Ban, S.S.; Alidina, H.M.; Okey, T.A.; Gregg, R.M.; Ban, N.C. Identifying potential marine climate change refugia: A case study in Canada’s Pacific marine ecosystems. Glob. Ecol. Conserv. 2016, 8, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Clark, M.R.; Schlacher, T.A.; Rowden, A.A.; Stocks, K.I.; Consalvey, M. Science priorities for seamounts: Research links to conservation and management. PLoS ONE 2012, 7, e29232. [Google Scholar] [CrossRef] [Green Version]
- Scales, K.L.; Miller, P.I.; Hawkes, L.A.; Ingram, S.N.; Sims, D.W.; Votier, S.C. Review: On the front line: Frontal zones as priority at-sea conservation areas for mobile marine vertebrates. J. Appl. Ecol. 2014, 51, 1575–1583. [Google Scholar] [CrossRef] [Green Version]
- Morato, T.; Hoyle, S.D.; Allain, V.; Nicol, S.J. Seamounts are hotspots of pelagic biodiversity in the open ocean. Proc. Natl. Acad. Sci. USA 2010, 107, 9707–9711. [Google Scholar] [CrossRef] [Green Version]
- Mantua, N.J.; Hare, S.R.; Zhang, Y.; Wallace, J.M.; Francis, R.C. A Pacific interdecadal climate oscillation with impacts on salmon production. Bull. Am. Meteorol. Soc. 1997, 78, 1069–1079. [Google Scholar] [CrossRef]
- Newman, M.; Alexander, M.A.; Ault, T.R.; Cobb, K.M.; Deser, C.; Di Lorenzo, E.; Mantua, N.J.; Miller, A.J.; Minobe, S.; Nakamura, H.; et al. The Pacific Decadal Oscillation, revisited. J. Clim. 2016, 29, 4399–4427. [Google Scholar] [CrossRef] [Green Version]
- Zwolinski, J.P.; Demer, D.A. Environmental and parental control of Pacific sardine (Sardinops sagax) recruitment. ICES J. Mar. Sci. 2014, 71, 2198–2207. [Google Scholar] [CrossRef] [Green Version]
- Litzow, M.A.; Ciannelli, L.; Puerta, P.; Wettstein, J.J.; Rykaczewski, R.R.; Opiekun, M. Non-stationary climate–salmon relationships in the Gulf of Alaska. Proceed. R. Soc. B Biol. Sci. 2018, 285, 20181855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantua, N.J.; Hare, S.R. The Pacific Decadal Oscillation. J. Oceanogr. 2002, 58, 35–44. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X.; Yi, Q.; Chen, Y. Influence of oceanic climate variability on stock level of western winter–spring cohort of Ommastrephes bartramii in the northwest Pacific Ocean. Int. J. Remote Sens. 2016, 37, 3974–3994. [Google Scholar] [CrossRef]
- Wessel, P.; Smith, W.H.F.; Scharroo, R.; Luis, J.; Wobbe, F. Generic mapping tools: Improved version released. Eos Trans. Am. Geophys. Union 2013, 94, 409–410. [Google Scholar] [CrossRef] [Green Version]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Biomod2: Ensemble Platform for Species Distribution Modeling. R Package Version 3.3-7. 2016. Available online: http://CRAN.R-project.org/package=biomod2 (accessed on 10 January 2016).
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Alabia, I.D.; Saitoh, S.-I.; Igarashi, H.; Ishikawa, Y.; Usui, N.; Kamachi, M.; Awaji, T.; Seito, M. Ensemble squid habitat model using three-dimensional ocean data. ICES J. Mar. Sci. 2016, 73, 1863–1874. [Google Scholar] [CrossRef] [Green Version]
- Scales, K.L.; Miller, P.I.; Ingram, S.N.; Hazen, E.L.; Bograd, S.J.; Phillips, R.A. Identifying predictable foraging habitats for a wide-ranging marine predator using ensemble ecological niche models. Divers. Distrib. 2016, 22, 212–224. [Google Scholar] [CrossRef] [Green Version]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (tss). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Chen, X.; Tian, S.; Chen, Y.; Liu, B. A modeling approach to identify optimal habitat and suitable fishing grounds for neon flying squid (Ommastrephes bartramii) in the northwest Pacific Ocean. Fish. Bull. 2010, 108, 14. [Google Scholar]
- Igarashi, H.; Ishikawa, Y.; Nishikawa, H.; Usui, N.; Sakai, M.; Saitoh, S.I.; Imamura, Y. Habitat Suitability Index Model for Neon Flying Squid Adding Its Stock Effect; Techno-Ocean: Kobe, Japan, 2016; pp. 449–453. [Google Scholar]
- Igarashi, H.; Saitoh, S.-I.; Ishikawa, Y.; Kamachi, M.; Usui, N.; Sakai, M.; Imamura, Y. Identifying potential habitat distribution of the neon flying squid (Ommastrephes bartramii) off the eastern coast of Japan in winter. Fish. Oceanogr. 2018, 27, 16–27. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhao, X.H.; Chen, Y. Influence of el niño/la niña on the western winter–spring cohort of neon flying squid (Ommastrephes bartramii) in the northwestern pacific ocean. ICES J. Mar. Sci. 2007, 64, 1152–1160. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Chen, X.; Yi, Q.; Chen, Y.; Zhang, Y. Variability of suitable habitat of western winter-spring cohort for neon flying squid in the northwest Pacific under anomalous environments. PLoS ONE 2015, 10, e0122997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kai, M.; Thorson, J.T.; Piner, K.R.; Maunder, M.N. Predicting the spatio-temporal distributions of pelagic sharks in the western and central North Pacific. Fish. Oceanogr. 2017, 26, 569–582. [Google Scholar] [CrossRef]
- Morato, T.; Hoyle, S.D.; Allain, V.; Nicol, S.J. Tuna longline fishing around west and central pacific seamounts. PLoS ONE 2011, 5, e14453. [Google Scholar] [CrossRef] [PubMed]
- Morato, T.; Varkey, D.A.; Damaso, C.; Machete, M.; Santos, M.; Prieto, R.; Pitcher, T.J.; Santos, R.S. Evidence of a seamount effect on aggregating visitors. Mar. Ecol. Prog. Ser. 2008, 357, 23–32. [Google Scholar] [CrossRef]
- Chiba, S.; Di Lorenzo, E.; Davis, A.; Keister, J.E.; Taguchi, B.; Sasai, Y.; Sugisaki, H. Large-scale climate control of zooplankton transport and biogeography in the Kuroshio-Oyashio extension region. Geophys. Res. Lett. 2013, 40, 5182–5187. [Google Scholar] [CrossRef]
- Macias, D.; Landry, M.R.; Gershunov, A.; Miller, A.J.; Franks, P.J.S. Climatic control of upwelling variability along the western north-american coast. PLoS ONE 2012, 7, e30436. [Google Scholar] [CrossRef] [Green Version]
- Chiba, S.; Sugisaki, H.; Nonaka, M.; Saino, T. Geographical shift of zooplankton communities and decadal dynamics of the Kuroshio–Oyashio currents in the western North Pacific. Glob. Chang. Biol. 2009, 15, 1846–1858. [Google Scholar] [CrossRef]
- Qiu, B.; Chen, S.; Schneider, N. Dynamical links between the decadal variability of the Oyashio and Kuroshio extensions. J. Clim. 2017, 30, 9591–9605. [Google Scholar] [CrossRef]
- Mugo, R.; Saitoh, S.-I.; Nihira, A.; Kuroyama, T. Habitat characteristics of skipjack tuna (Katsuwonus pelamis) in the western North Pacific: A remote sensing perspective. Fish. Oceanogr. 2010, 19, 382–396. [Google Scholar] [CrossRef]
- Zainuddin, M.; Kiyofuji, H.; Saitoh, K.; Saitoh, S.-I. Using multi-sensor satellite remote sensing and catch data to detect ocean hot spots for albacore (Thunnus alalunga) in the northwestern North Pacific. Deep Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 419–431. [Google Scholar] [CrossRef]
- Yatsu, A.; Chiba, S.; Yamanaka, Y.; Ito, S.-I.; Shimizu, Y.; Kaeriyama, M.; Watanabe, Y. Climate forcing and the Kuroshio/Oyashio ecosystem. ICES J. Mar. Sci. 2013, 70, 922–933. [Google Scholar] [CrossRef] [Green Version]
- Braun, C.D.; Gaube, P.; Sinclair-Taylor, T.H.; Skomal, G.B.; Thorrold, S.R. Mesoscale eddies release pelagic sharks from thermal constraints to foraging in the ocean twilight zone. Proc. Natl. Acad. Sci. USA 2019, 116, 17187. [Google Scholar] [CrossRef] [Green Version]
- Ayers, J.M.; Lozier, M.S. Physical controls on the seasonal migration of the North Pacific transition zone chlorophyll front. J. Geophys. Res. Oceans 2010, 115, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Polovina, J.J.; Howell, E.; Kobayashi, D.R.; Seki, M.P. The transition zone chlorophyll front, a dynamic global feature defining migration and forage habitat for marine resources. Prog. Oceanogr. 2001, 49, 469–483. [Google Scholar] [CrossRef]
- Polovina, J.J.; Howell, E.A.; Kobayashi, D.R.; Seki, M.P. The transition zone chlorophyll front updated: Advances from a decade of research. Prog. Oceanogr. 2017, 150, 79–85. [Google Scholar] [CrossRef]
- Pecl, G.; Jackson, G. The potential impacts of climate change on inshore squid: Biology, ecology and fisheries. Rev. Fish. Biol. Fish. 2008, 18, 373–385. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; García Molinos, J.; Halpern, B.S.; Hoegh-Guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, D.S.; et al. Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 2016, 3, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Guraslan, C.; Fach, B.A.; Oguz, T. Understanding the impact of environmental variability on anchovy overwintering migration in the black sea and its implications for the fishing industry. Front. Mar. Sci. 2017, 4, 275. [Google Scholar] [CrossRef] [Green Version]
- Alabia, I.D.; García Molinos, J.; Saitoh, S.-I.; Hirawake, T.; Hirata, T.; Mueter, F.J. Distribution shifts of marine taxa in the Pacific Arctic under contemporary climate changes. Divers. Distrib. 2018, 24, 1583–1597. [Google Scholar] [CrossRef] [Green Version]
- Townsend, M.; Davies, K.; Hanley, N.; Hewitt, J.E.; Lundquist, C.J.; Lohrer, A.M. The challenge of implementing the marine ecosystem service concept. Front. Mar. Sci. 2018, 5, 359. [Google Scholar] [CrossRef] [Green Version]
- Hazen, E.L.; Abrahms, B.; Brodie, S.; Carroll, G.; Jacox, M.G.; Savoca, M.S.; Scales, K.L.; Sydeman, W.J.; Bograd, S.J. Marine top predators as climate and ecosystem sentinels. Front. Ecol. Environ. 2019, 17, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Tittensor, D.P.; Beger, M.; Boerder, K.; Boyce, D.G.; Cavanagh, R.D.; Cosandey-Godin, A.; Crespo, G.O.; Dunn, D.C.; Ghiffary, W.; Grant, S.M.; et al. Integrating climate adaptation and biodiversity conservation in the global ocean. Sci. Adv. 2019, 5, 9969. [Google Scholar] [CrossRef] [Green Version]







| Model Algorithm | Abbrev | Model Parameterization Settings | R Packages |
|---|---|---|---|
| Generalized linear model | GLM | family = ‘binomial’ (link = ‘logit’), type = ‘quadratic’ | stats ver. 3.4.4 |
| Generalized additive model | GAM | family = ‘binomial’ (link = ‘logit’), type = ‘s_smoother’, | mgcv ver. 1.8-23 |
| Boosted Regression Trees | GBM | distribution = ‘bernoulli’, number of trees = 2500, shrinkage = 0.001, bag fraction = 0.5, train fraction = 1, cross-validation folds = 3 | gbm ver. 2.1.3 |
| Classification Tree Analysis | CTA | method = class, complexity parameter = 0.001, cross-validation folds = 5 | rpart ver. 4.1–13 |
| Artificial Neural Network | ANN | maximum iteration = 200, cross-validation folds = 5, random weight = 0.1 | nnet ver. 7.3–12 |
| Surface Range Envelop | SRE | tolerance boundary cut-off = 0.025 | biomod2 3.3–7 |
| Flexible Discriminant Analysis | FDA | regression method = ‘mars’ | biomod2 3.3–7 |
| Multiple Adaptive Regression Splines | MARS | maximum interaction degree = 2, penalty = 2, forward stepwise stopping threshold = 0.001, prune feature enabled | biomod2 3.3–7 |
| Random Forest | RF | number of trees = 500, node size = 5 | biomod2 3.3–7 |
| Maximum Entropy | MaxEnt | maximum training iterations = 200, linear/quadratic/product/hinge features enabled, default prevalence = 0.5 | MaxEnt ver. 3.4.0 |
| Species Distribution Models (SDMs) | Abbreviations | Model Performance Metrics | ||
|---|---|---|---|---|
| AUC | Kappa | TSS | ||
| Generalized additive model | GAM | 0.952 | 0.646 | 0.810 |
| Generalized linear model | GLM | 0.950 | 0.644 | 0.803 |
| Generalized boosting model | GBM | 0.957 | 0.659 | 0.822 |
| Artificial neural network | ANN | 0.955 | 0.654 | 0.814 |
| Random forest | RF | 0.955 | 0.651 | 0.810 |
| Maximum entropy | MaxEnt | 0.954 | 0.641 | 0.801 |
| Classification tree analysis | CTA | 0.924 | 0.637 | 0.807 |
| Surface range envelope | SRE | 0.888 | 0.603 | 0.776 |
| Flexible discriminant analysis | FDA | 0.945 | 0.617 | 0.785 |
| Multivariate adaptive regression splines | MARS | 0.952 | 0.648 | 0.810 |
| PDO Phase Transitions | Categories | Large Decrease | Small Decrease | Largely Unchanged | Small Increase | Large Increase | |
|---|---|---|---|---|---|---|---|
| Variables (Unit) | 0% | 20% | 40% | 60% | 80% | 100% | |
| Cold-Warm (T1) | Habitat (HSI) | −0.0928 | −0.0133 | −0.0002 | 0.0061 | 0.0290 | 0.1339 |
| SST (°C/yr) | −2.8460 | −0.5805 | 0.0252 | 0.3311 | 0.6211 | 2.9051 | |
| SSH (cm/yr) | −0.3578 | −0.1093 | 0.0263 | 0.1042 | 0.1822 | 0.3739 | |
| Warm-Cold (T2) | Habitat (HSI) | −0.0943 | −0.0199 | 0.0000 | 0.0036 | 0.0240 | 0.0712 |
| SST (°C/yr) | −1.4725 | 0.0996 | 0.3072 | 0.4463 | 0.6537 | 3.0849 | |
| SSH (cm/yr) | −0.0983 | 0.0388 | 0.0967 | 0.1569 | 0.2384 | 0.3722 | |
| Environmental Factors | Optimal Ranges | Total Area (1 × 106 km2) | ||
|---|---|---|---|---|
| P1 (1999–2001) | P2 (2002–2006) | P3 (2007–2013) | ||
| SST | 12–17 °C | 1.96 | 1.85 | 1.98 |
| SSH | 40–120 cm | 6.26 | 6.00 | 6.37 |
| PDO-Based Climatic Periods | Ranges (Minimum, Maximum) | HSI Mean ± 1SD | Total Area (1 × 105 km2) | ||
|---|---|---|---|---|---|
| Longitude | Latitude | HSI | |||
| P1 (1999–2001) | 147.25, 200.00 | 40.00, 44.50 | 0.50, 0.90 | 0.70 ± 0.11 | 6.93 |
| P2 (2002–2006) | 154.00, 200.00 | 40.00, 44.00 | 0.50, 0.92 | 0.72 ± 0.12 | 6.87 |
| P3 (2007–2013) | 168.75, 200.00 | 40.75, 44.75 | 0.50, 0.87 | 0.67 ± 0.09 | 7.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabia, I.D.; Saitoh, S.-I.; Igarashi, H.; Ishikawa, Y.; Imamura, Y. Spatial Habitat Shifts of Oceanic Cephalopod (Ommastrephes bartramii) in Oscillating Climate. Remote Sens. 2020, 12, 521. https://doi.org/10.3390/rs12030521
Alabia ID, Saitoh S-I, Igarashi H, Ishikawa Y, Imamura Y. Spatial Habitat Shifts of Oceanic Cephalopod (Ommastrephes bartramii) in Oscillating Climate. Remote Sensing. 2020; 12(3):521. https://doi.org/10.3390/rs12030521
Chicago/Turabian StyleAlabia, Irene D., Sei-Ichi Saitoh, Hiromichi Igarashi, Yoichi Ishikawa, and Yutaka Imamura. 2020. "Spatial Habitat Shifts of Oceanic Cephalopod (Ommastrephes bartramii) in Oscillating Climate" Remote Sensing 12, no. 3: 521. https://doi.org/10.3390/rs12030521
APA StyleAlabia, I. D., Saitoh, S.-I., Igarashi, H., Ishikawa, Y., & Imamura, Y. (2020). Spatial Habitat Shifts of Oceanic Cephalopod (Ommastrephes bartramii) in Oscillating Climate. Remote Sensing, 12(3), 521. https://doi.org/10.3390/rs12030521