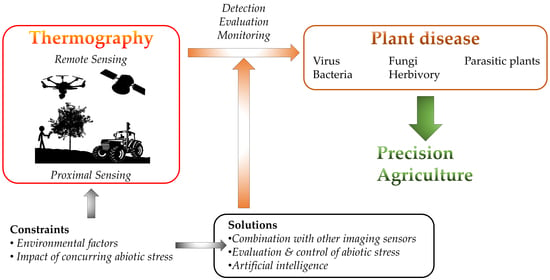
Thermal Imaging for Plant Stress Detection and Phenotyping †
Abstract
:1. Introduction
- Normalized canopy or leaf temperature with reference to air temperature (ΔT), utilized as an index of crop water status. The applications of ΔT was recently reviewed by Still et al. [15];
- Crop water stress index (CSWI), which introduces two baselines: (Tcanopy − Tair)wet as the estimated difference for a well-watered plant, and (Tcanopy − Tair)dry for a dry (non-transpiring) plant. CWSI is one of the most commonly used normalization methods for TIR measurements, which overcomes the effects of other environmental parameters affecting plant temperature [16,17];
- Index of stomatal conductance (IG), since it is directly proportional to this parameter [18];
- Normalized relative canopy temperature (NRCT), based on the maximum and the minimum temperature measured in the whole field trial. This parameter has been found to be a valid estimation of the crop water status [22];
- Average canopy temperature (Tav), based on maximum and minimum values of temperature, was one of the first parameters used by the conventional infrared thermography [23,24]. However, this parameter can excessively simplify outcome data, and some important thermal information can be lost. Estimated shape factors derived by fitting the whole temperature data of the thermal images to the Weibull distribution could solve this constraint [25].
| TIR Stress Index | Formula | Ref. |
|---|---|---|
| ΔT, normalized canopy or leaf temperature | [26] | |
| CWSI, crop water stress index | [17,27] | |
| IG, index of stomatal conductance | [18] | |
| MTD, maximum temperature difference | [19] | |
| NRCT, normalized relative canopy temperature | [22] | |
| Tav, average canopy temperature | [24] |
| TIR SI | Physiol. Param. | Plant Species | Scale | Ref. |
|---|---|---|---|---|
| ΔT | gs | Papaya | PS | [28] |
| Vineyard, corn, olive, citrus, poplar, almond, apple, persimmon | RS | [29,30,31,32,33,34,35,36,37,38] | ||
| Em | Papaya | PS | [28] | |
| AN | Papaya | PS | [28] | |
| Ψ | Vineyard, olive, citrus, almond, Prunus sp., persimmon, apple | RS | [29,32,33,34,35,38,39,40,41,42,43] | |
| CWSI | gs | Fava bean, spinach | PS | [44,45] |
| Vineyard, olive, potato, almond, pistachio | RS | [29,31,38,46,47,48,49,50,51,52,53,54,55,56,57] | ||
| Ψ | Vineyard, Prunus sp., almond, cotton, olive, citrus, pistachio | RS | [29,38,40,43,48,49,53,55,56,57,58,59] | |
| Em | Olive | RS | [53] | |
| SM | Corn | PS | [60] | |
| IG | gs | Fava bean | PS | [44] |
| Vineyard | RS | [23,29,38,46,50,51,52,57] | ||
| Ψ | Vineyard | RS | [29,38,57] | |
| NRCT | RWC | Wheat | PS | [61] |
| CWC | Wheat | PS | [61] | |
| Tav | gs | Vineyard | RS | [46] |
| Tcanopy | gs | Vineyard, citrus, almond | RS | [29,52,55,62,63] |
| Ψ | Vineyard, cotton, citrus, almond | RS | [29,55,63,64] | |
| SM | Soybean | RS | [65] | |
| Tleaf | gs | Papaya, barley, wheat, rice | PS | [28,66,67] |
| Em | Papaya | PS | [28] | |
| AN | Papaya | PS | [28] |
2. Biotic Stress Detection by Thermography at Different Scales
2.1. Proximal Sensing on Growth Chambers and Greenhouses
2.1.1. Viral Infections
2.1.2. Bacterial Infections
2.1.3. Interactions with Pathogenic Fungi and Oomycetes
2.1.4. Herbivory and Parasitic Plants
2.2. Remote Sensing on Crop Fields
3. Assessing of Plant Abiotic Stress by Thermography
3.1. Proximal Sensing on Growth Chambers and Greenhouses
3.2. Remote Sensing on Crop Fields
4. Future Perspectives
4.1. Future Technical Development of Thermography
4.2. Strategies to Overcome the Intrinsic Non-Specificity of Thermal Patterns
4.3. Uses of Plant Thermography for Agriculture in the Near Future
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Stevanović, M.; Popp, A.; Lotze-Campen, H.; Dietrich, J.P.; Müller, C.; Bonsch, M.; Schmitz, C.; Bodirsky, B.L.; Humpenöder, F.; Weindl, I. The impact of high-end climate change on agricultural welfare. Sci. Adv. 2016, 2, e1501452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvajal-Yepes, M.; Cardwell, K.; Nelson, A.; Garrett, K.A.; Giovani, B.; Saunders, D.G.O.; Kamoun, S.; Legg, J.P.; Verdier, V.; Lessel, J.; et al. A global surveillance system for crop diseases. Science 2019, 364, 1237–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, J.; Thrall, P.H.; Papaïx, J.; Xie, L.; Burdon, J.J. Playing on a Pathogen’s Weakness: Using Evolution to Guide Sustainable Plant Disease Control Strategies. Annu. Rev. Phytopathol. 2015, 53, 19–43. [Google Scholar] [CrossRef] [PubMed]
- Mahlein, A.-K. Plant disease detection by imaging sensors—Parallels and specific demands for precision agriculture and plant phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, H. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology, 3rd ed.; Cambridge University Press: Cambridge, UK, 2014; Volume 56. [Google Scholar]
- Prashar, A.; Yildiz, J.; McNicol, J.W.; Bryan, G.J.; Jones, H.G. Infra-red thermography for high throughput field phenotyping in Solanum tuberosum. PLoS ONE 2013, 8, e65816. [Google Scholar] [CrossRef] [PubMed]
- Milthorpe, F.L.; Spencer, E.J. Experimental studies of the factors controlling transpiration. J. Exp. Bot. 1957, 8, 413–437. [Google Scholar] [CrossRef]
- Scarth, G.W.; Loewy, A.; Shaw, M. Use of the infrared total absorption method for estimating the time course of photosynthesis and transpiration. Can. J. Res. 1948, 26c, 94–107. [Google Scholar] [CrossRef]
- Fuchs, M.; Tanner, C.B. Infrared thermometry of vegetation. Agron. J. 1966, 58, 597–601. [Google Scholar] [CrossRef]
- Jones, H.G. Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plantcell Environ. 1999, 22, 1043–1055. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.G. Application of thermal imaging and infrared sensing in plant physiology and ecophysiology. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2004; Volume 41, pp. 107–163. [Google Scholar]
- Ishimwe, R.; Abutaleb, K.; Ahmed, F. Applications of thermal imaging in agriculture—A review. Adv. Remote Sens. 2014, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Khanal, S.; Fulton, J.; Shearer, S. An overview of current and potential applications of thermal remote sensing in precision agriculture. Comput. Electron. Agric. 2017, 139, 22–32. [Google Scholar] [CrossRef]
- Chaerle, L.; Van der Straeten, D. Seeing is believing: Imaging techniques to monitor plant health. Biochim. Biophys. Acta 2001, 1519, 153–166. [Google Scholar] [CrossRef]
- Still, C.; Powell, R.; Aubrecht, D.; Kim, Y.; Helliker, B.; Roberts, D.; Richardson, A.D.; Goulden, M. Thermal imaging in plant and ecosystem ecology: Applications and challenges. Ecosphere 2019, 10, e02768. [Google Scholar] [CrossRef] [Green Version]
- Idso, S.B.; Jackson, R.D.; Pinter, P.J.; Reginato, R.J.; Hatfield, J.L. Normalizing the stress-degree-day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Jones, H.G. Use of infrared thermometry for estimation of stomatal conductance as a possible aid to irrigation scheduling. Agric. For. Meteorol. 1999, 95, 139–149. [Google Scholar] [CrossRef]
- Lindenthal, M.; Steiner, U.; Dehne, H.W.; Oerke, E.C. Effect of downy mildew development on transpiration of cucumber leaves visualized by digital infrared thermography. Phytopathology 2005, 95, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Saglam, A.; Chaerle, L.; Van Der Straeten, D.; Valcke, R. Promising monitoring techniques for plant science: Thermal and chlorophyll fluorescence imaging. In Photosynthesis, Productivity and Environmental Stress; Wiley: Hoboken, NJ, USA, 2019; pp. 241–266. [Google Scholar] [CrossRef]
- Simko, I.; Jiménez-Berni, J.A.; Sirault, X.R.R. Phenomic approaches and tools for phytopathologists. Phytopathology 2017, 107, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, S.; Rischbeck, P.; Schmidhalter, U. Comparing the performance of active and passive reflectance sensors to assess the normalized relative canopy temperature and grain yield of drought-stressed barley cultivars. Field Crop. Res. 2015, 177, 148–160. [Google Scholar] [CrossRef]
- Jones, H.G.; Stoll, M.; Santos, T.; de Sousa, C.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef]
- Heermann, D.; Duke, H. Electrical load and water management. Proc. Annu. Tech. Conf. 1978, 1978, 60–67. [Google Scholar]
- Mastrodimos, N.; Lentzou, D.; Templalexis, C.; Tsitsigiannis, D.I.; Xanthopoulos, G. Development of thermography methodology for early diagnosis of fungal infection in table grapes: The case of Aspergillus carbonarius. Comput. Electron. Agric. 2019, 165, 104972. [Google Scholar] [CrossRef]
- Idso, S.B.; Jackson, R.D.; Reginato, R.J. Remote-sensing of crop yields. Science 1977, 196, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Idso, S.B.; Reginato, R.J.; Jackson, R.D.; Pinter, P.J. Foliage and air temperatures: Evidence for a dynamic “equivalence point”. Agric. Meteorol. 1981, 24, 223–226. [Google Scholar] [CrossRef]
- Lima, R.; García-Tejero, I.; Lopes, T.; Costa, J.; Vaz, M.; Durán-Zuazo, V.; Chaves, M.; Glenn, D.; Campostrini, E. Linking thermal imaging to physiological indicators in Carica papaya L. under different watering regimes. Agric. Water Manag. 2016, 164, 148–157. [Google Scholar] [CrossRef]
- Baluja, J.; Diago, M.P.; Balda, P.; Zorer, R.; Meggio, F.; Morales, F.; Tardaguila, J. Assessment of vineyard water status variability by thermal and multispectral imagery using an unmanned aerial vehicle (UAV). Irrig. Sci. 2012, 30, 511–522. [Google Scholar] [CrossRef]
- Jiménez-Berni, J.A.; Zarco-Tejada, P.J.; Suárez, L.; Fereres, E. Thermal and narrowband multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle. IEEE Trans. Geosci. Remote 2009, 47, 722–738. [Google Scholar] [CrossRef] [Green Version]
- Ben-Gal, A.; Agam, N.; Alchanatis, V.; Cohen, Y.; Yermiyahu, U.; Zipori, I.; Presnov, E.; Sprintsin, M.; Dag, A. Evaluating water stress in irrigated olives: Correlation of soil water status, tree water status, and thermal imagery. Irrig. Sci. 2009, 27, 367–376. [Google Scholar] [CrossRef]
- Ballester, C.; Castel, J.; Jimenez-Bello, M.A.; Castel, J.R.; Intrigliolo, D.S. Thermographic measurement of canopy temperature is a useful tool for predicting water deficit effects on fruit weight in citrus trees. Agric. Water Manag. 2013, 122, 1–6. [Google Scholar] [CrossRef]
- Ballester, C.; Jimenez-Bello, M.A.; Castel, J.R.; Intrigliolo, D.S. Usefulness of thermography for plant water stress detection in citrus and persimmon trees. Agric. For. Meteorol. 2013, 168, 120–129. [Google Scholar] [CrossRef]
- García-Tejero, I.; Durán-Zuazo, V.H.; Arriaga, J.; Hernández, A.; Vélez, L.M.; Muriel-Fernández, J.L. Approach to assess infrared thermal imaging of almond trees under water-stress conditions. Fruits 2012, 67, 463–474. [Google Scholar] [CrossRef]
- González-Dugo, V.; Zarco-Tejada, P.; Jiménez-Berni, J.A.; Suárez, L.; Goldhamer, D.; Fereres, E. Almond tree canopy temperature reveals intra-crown variability that is water stress-dependent. Agric. For. Meteorol. 2012, 154–155, 156–165. [Google Scholar] [CrossRef]
- Coupel-Ledru, A.; Pallas, B.; Delalande, M.; Boudon, F.; Carrie, E.; Martinez, S.; Regnard, J.L.; Costes, E. Multi-scale high-throughput phenotyping of apple architectural and functional traits in orchard reveals genotypic variability under contrasted watering regimes. Hortic. Res. Engl. 2019, 6, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludovisi, R.; Tauro, F.; Salvati, R.; Khoury, S.; Mugnozza, G.S.; Harfouche, A. UAV-based thermal imaging for high-throughput field phenotyping of black poplar response to drought. Front. Plant Sci. 2017, 8, 18. [Google Scholar] [CrossRef]
- Pagay, V.; Kidman, C.M. Evaluating remotely-sensed grapevine (Vitis vinifera L.) water stress responses across a viticultural region. Agronomy 2019, 9, 682. [Google Scholar] [CrossRef] [Green Version]
- Sepulcre-Cantó, G.; Zarco-Tejada, P.J.; Jiménez-Muñoz, J.C.; Sobrino, J.A.; de Miguel, E.; Villalobos, F.J. Detection of water stress in an olive orchard with thermal remote sensing imagery. Agric. For. Meteorol. 2006, 136, 31–44. [Google Scholar] [CrossRef]
- González-Dugo, V.; Zarco-Tejada, P.; Nicolas, E.; Nortes, P.A.; Alarcón, J.J.; Intrigliolo, D.S.; Fereres, E. Using high resolution UAV thermal imagery to assess the variability in the water status of five fruit tree species within a commercial orchard. Precis. Agric. 2013, 14, 660–678. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Hernández, A.; Rodríguez, V.; Ponce, J.; Ramos, V.; Muriel, J.; Durán Zuazo, V. Estimating almond crop coefficients and physiological response to water stress in semiarid environments (SW Spain). J. Agric. Sci. Technol. 2015, 17, 1255–1266. [Google Scholar]
- Gómez-Candón, D.; Virlet, N.; Labbé, S.; Jolivot, A.; Regnard, J.-L. Field phenotyping of water stress at tree scale by UAV-sensed imagery: New insights for thermal acquisition and calibration. Precis. Agric. 2016, 17, 786–800. [Google Scholar] [CrossRef]
- Blaya-Ros, P.J.; Blanco, V.; Domingo, R.; Soto-Valles, F.; Torres-Sánchez, R. Feasibility of low-cost thermal imaging for monitoring water stress in young and mature sweet cherry trees. Appl. Sci. 2020, 10, 5461. [Google Scholar] [CrossRef]
- Leinonen, I.; Jones, H.G. Combining thermal and visible imagery for estimating canopy temperature and identifying plant stress. J. Exp. Bot. 2004, 55, 1423–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masseroni, D.; Ortuani, B.; Corti, M.; Gallina, P.M.; Cocetta, G.; Ferrante, A.; Facchi, A. Assessing the reliability of thermal and optical imaging techniques for detecting crop water status under different nitrogen levels. Sustainability 2017, 9, 1548. [Google Scholar] [CrossRef] [Green Version]
- Grant, O.M.; Tronina, L.; Jones, H.G.; Chaves, M.M. Exploring thermal imaging variables for the detection of stress responses in grapevine under different irrigation regimes. J. Exp. Bot. 2007, 58, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Alchanatis, V.; Cohen, Y.; Meron, M.; Tsipris, J.; Naor, A.; Ostrovsky, V.; Sprintsin, M.; Cohen, S. Use of thermal and visible imagery for estimating crop water status of irrigated grapevine. J. Exp. Bot. 2007, 58, 827–838. [Google Scholar] [CrossRef] [Green Version]
- Sepúlveda-Reyes, D.; Ingram, B.; Bardeen, M.; Zúñiga, M.; Ortega-Farías, S.; Poblete-Echeverría, C. Selecting canopy zones and thresholding approaches to assess grapevine water status by using aerial and ground-based thermal imaging. Remote Sens. 2016, 8, 822. [Google Scholar] [CrossRef] [Green Version]
- Santesteban, L.G.; Di Gennaro, S.F.; Herrero-Langreo, A.; Miranda, C.; Royo, J.B.; Matese, A. High-resolution UAV-based thermal imaging to estimate the instantaneous and seasonal variability of plant water status within a vineyard. Agric. Water Manag. 2017, 183, 49–59. [Google Scholar] [CrossRef]
- Gago, J.; Martorell, S.; Tomás, M.; Pou, A.; Millán, B.; Ramón, J.; Ruiz, M.; Sánchez, R.; Galmés, J.; Conesa, M. High-resolution aerial thermal imagery for plant water status assessment in vineyards using a multicopter-RPAS. In Proceedings of the VII Congreso Ibérico de Agroingenieria y Ciencias Hortícolas, Madrid, Spain, 26–29 August 2013. [Google Scholar]
- Pou, A.; Diago, M.P.; Medrano, H.; Baluja, J.; Tardaguila, J. Validation of thermal indices for water status identification in grapevine. Agric. Water Manag. 2014, 134, 60–72. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Costa, J.M.; Egipto, R.; Durán-Zuazo, V.H.; Lima, R.S.N.; Lopes, C.M.; Chaves, M.M. Thermal data to monitor crop-water status in irrigated Mediterranean viticulture. Agric. Water Manag. 2016, 176, 80–90. [Google Scholar] [CrossRef]
- Egea, G.; Padilla-Díaz, C.M.; Martinez-Guanter, J.; Fernández, J.E.; Pérez-Ruiz, M. Assessing a crop water stress index derived from aerial thermal imaging and infrared thermometry in super-high density olive orchards. Agric. Water Manag. 2017, 187, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Rud, R.; Cohen, Y.; Alchanatis, V.; Levi, A.; Brikman, R.; Shenderey, C.; Heuer, B.; Markovitch, T.; Dar, Z.; Rosen, C.; et al. Crop water stress index derived from multi-year ground and aerial thermal images as an indicator of potato water status. Precis. Agric. 2014, 15, 273–289. [Google Scholar] [CrossRef]
- García-Tejero, I.; Rubio, A.; Viñuela, I.; Hernández, A.; Gutiérrez-Gordillo, S.; Rodríguez-Pleguezuelo, C.; Durán-Zuazo, V. Thermal imaging at plant level to assess the crop-water status in almond trees (cv. Guara) under deficit irrigation strategies. Agric. Water Manag. 2018, 208, 176–186. [Google Scholar] [CrossRef]
- González-Dugo, V.; Goldhamer, D.; Zarco-Tejada, P.; Fereres, E. Improving the precision of irrigation in a pistachio farm using an unmanned airborne thermal system. Irrig. Sci. 2015, 33, 43–52. [Google Scholar] [CrossRef]
- Belfiore, N.; Vinti, R.; Lovat, L.; Chitarra, W.; Tomasi, D.; De Bei, R.; Meggio, F.; Gaiotti, F. Infrared thermography to estimate vine water status: Optimizing canopy measurements and thermal indices for the varieties merlot and moscato in northern Italy. Agronomy 2019, 9, 821. [Google Scholar] [CrossRef] [Green Version]
- Cohen, Y.; Alchanatis, V.; Meron, M.; Saranga, Y.; Tsipris, J. Estimation of leaf water potential by thermal imagery and spatial analysis. J. Exp. Bot. 2005, 56, 1843–1852. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Berni, J.A.; Zarco-Tejada, P.J.; Sepulcre-Cantó, G.; Fereres, E.; Villalobos, F. Mapping canopy conductance and CWSI in olive orchards using high resolution thermal remote sensing imagery. Remote Sens. Environ. 2009, 113, 2380–2388. [Google Scholar] [CrossRef]
- Mangus, D.L.; Sharda, A.; Zhang, N. Development and evaluation of thermal infrared imaging system for high spatial and temporal resolution crop water stress monitoring of corn within a greenhouse. Comput. Electron. Agric. 2016, 121, 149–159. [Google Scholar] [CrossRef]
- Elsayed, S.; Elhoweity, M.; Ibrahim, H.H.; Dewir, Y.H.; Migdadi, H.M.; Schmidhalter, U. Thermal imaging and passive reflectance sensing to estimate the water status and grain yield of wheat under different irrigation regimes. Agric. Water Manag. 2017, 189, 98–110. [Google Scholar] [CrossRef]
- Zúñiga-Espinoza, C.; Khot, L.R.; Sankaran, S.; Jacoby, P.W. High resolution multispectral and thermal remote sensing-based water stress assessment in subsurface irrigated grapevines. Remote Sens. 2017, 9, 961. [Google Scholar] [CrossRef] [Green Version]
- Zarco-Tejada, P.J.; González-Dugo, V.; Jiménez-Berni, J.A. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Padhi, J.; Misra, R.K.; Payero, J.O. Estimation of soil water deficit in an irrigated cotton field with infrared thermography. Field Crop. Res. 2012, 126, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Crusiol, L.G.T.; Nanni, M.R.; Furlanetto, R.H.; Sibaldelli, R.N.R.; Cezar, E.; Mertz-Henning, L.M.; Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B. UAV-based thermal imaging in the assessment of water status of soybean plants. Int. J. Remote Sens. 2020, 41, 3243–3265. [Google Scholar] [CrossRef]
- Siddiqui, Z.S.; Cho, J.-I.; Park, S.-H.; Kwon, T.-R.; Lee, G.-S.; Jeong, M.-J.; Kim, K.-W.; Lee, S.-K.; Park, S.-C. Phenotyping of rice in salt stress environment using high-throughput infrared imaging. Acta Bot. Croat. 2014, 73, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Sirault, X.R.R.; James, R.A.; Furbank, R.T. A new screening method for osmotic component of salinity tolerance in cereals using infrared thermography. Funct. Plant Biol. 2009, 36, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Melotto, M.; He, S.Y. Plant stomata: A checkpoint of host immunity and pathogen virulence. Curr. Opin. Biotechnol. 2010, 21, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Roitsch, T.; Cabrera-Bosquet, L.; Fournier, A.; Ghamkhar, K.; Jiménez-Berni, J.A.; Pinto, F.; Ober, E.S. Review: New sensors and data-driven approaches—A path to next generation phenomics. Plant Sci. 2019, 282, 2–10. [Google Scholar] [CrossRef]
- Sperschneider, J. Machine learning in plant–pathogen interactions: Empowering biological predictions from field scale to genome scale. New Phytol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Ganapathysubramanian, B.; Singh, A.K.; Sarkar, S. Machine learning for high-throughput stress phenotyping in plants. Trends Plant Sci. 2016, 21, 110–124. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Luo, Z.; Zhang, W.; Lv, Z.; Xu, Y. Deep learning application in plant stress imaging: A review. AgriEngineering 2020, 2, 430–446. [Google Scholar] [CrossRef]
- Liakos, K.G.; Busato, P.; Moshou, D.; Pearson, S.; Bochtis, D. Machine learning in agriculture: A review. Sensors 2018, 18, 2674. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.G. Thermal imaging and infrared sensing in plant ecophysiology. In Advances in Plant Ecophysiology Techniques; Springer: Berlin, Germany, 2018; pp. 135–151. [Google Scholar] [CrossRef]
- Vialet-Chabrand, S.; Lawson, T. Dynamic leaf energy balance: Deriving stomatal conductance from thermal imaging in a dynamic environment. J. Exp. Bot. 2019, 70, 2839–2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, G.; Modica, G. Applications of UAV thermal imagery in precision agriculture: State of the art and future research outlook. Remote Sens. 2020, 12, 1491. [Google Scholar] [CrossRef]
- Prashar, A.; Jones, H.G. Infra-red thermography as a high-throughput tool for field phenotyping. Agronomy 2014, 4, 397–417. [Google Scholar] [CrossRef]
- Kelly, J.; Kljun, N.; Olsson, P.-O.; Mihai, L.; Liljeblad, B.; Weslien, P.; Klemedtsson, L.; Eklundh, L. Challenges and best practices for deriving temperature data from an uncalibrated UAV thermal infrared camera. Remote Sens. 2019, 11, 567. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring plant diseases and pests through remote sensing technology: A review. Comput. Electron. Agric. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Sagan, V.; Maimaitijiang, M.; Sidike, P.; Eblimit, K.; Peterson, K.T.; Hartling, S.; Esposito, F.; Khanal, K.; Newcomb, M.; Pauli, D. UAV-based high resolution thermal imaging for vegetation monitoring, and plant phenotyping using ICI 8640 P, FLIR Vue Pro R 640, and thermoMap cameras. Remote Sens. 2019, 11, 330. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.M.; Marques da Silva, J.; Pinheiro, C.; Barón, M.; Mylona, P.; Centritto, M.; Haworth, M.; Loreto, F.; Uzilday, B.; Turkan, I.; et al. Opportunities and limitations of crop phenotyping in southern European countries. Front. Plant Sci. 2019, 10, 1125. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Guo, R.; Li, M.; Chen, Y.; Li, G. A review of computer vision technologies for plant phenotyping. Comput. Electron. Agric. 2020, 176, 105672. [Google Scholar] [CrossRef]
- Zhang, C.; Valente, J.; Kooistra, L.; Guo, L.; Wang, W. Opportunities of UAVs in orchard management. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, XLII-2/W13, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Maes, W.H.; Steppe, K. Perspectives for remote sensing with unmanned aerial vehicles in precision agriculture. Trends Plant Sci. 2019, 24, 152–164. [Google Scholar] [CrossRef]
- Barbedo, J.G.A. A review on the use of unmanned aerial vehicles and imaging sensors for monitoring and assessing plant stresses. Drones 2019, 3, 40. [Google Scholar] [CrossRef] [Green Version]
- Sawinski, K.; Mersmann, S.; Robatzek, S.; Bohmer, M. Guarding the green: Pathways to stomatal immunity. Mol. Plant Microbe Ineract. 2013, 26, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Agurla, S.; Raghavendra, A.S. Convergence and divergence of signaling events in guard cells during stomatal closure by plant hormones or microbial elicitors. Front. Plant Sci. 2016, 7, 1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barón, M.; Pineda, M.; Pérez-Bueno, M.L. Picturing pathogen infection in plants. Z. Naturforsch. C Bio. Sci. 2016, 71, 355–368. [Google Scholar] [CrossRef]
- Grimmer, M.K.; John Foulkes, M.; Paveley, N.D. Foliar pathogenesis and plant water relations: A review. J. Exp. Bot. 2012, 63, 4321–4331. [Google Scholar] [CrossRef] [Green Version]
- Smigaj, M.; Gaulton, R.; Suárez, J.C.; Barr, S.L. Canopy temperature from an Unmanned Aerial Vehicle as an indicator of tree stress associated with red band needle blight severity. For. Ecol. Manag. 2019, 433, 699–708. [Google Scholar] [CrossRef]
- Montero, R.; Pérez-Bueno, M.L.; Barón, M.; Florez-Sarasa, I.; Tohge, T.; Fernie, A.R.; El Aou Ouad, H.; Flexas, J.; Bota, J. Alterations in primary and secondary metabolism in Vitis vinifera ‘Malvasía de Banyalbufar’ upon infection with Grapevine leafroll associated virus 3 (GLRaV-3). Physiol. Plant. 2016, 157, 442–452. [Google Scholar] [CrossRef]
- Chaerle, L.; Hagenbeek, D.; De Bruyne, E.; Valcke, R.; Van der Straeten, D. Thermal and chlorophyll-fluorescence imaging distinguish plant-pathogen interactions at an early stage. Plant Cell Physiol. 2004, 45, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Chaerle, L.; Pineda, M.; Romero-Aranda, R.; Van der Straeten, D.; Barón, M. Robotized thermal and chlorophyll fluorescence imaging of Pepper mild mottle virus infection in Nicotiana benthamiana. Plant Cell Physiol. 2006, 47, 1323–1336. [Google Scholar] [CrossRef] [Green Version]
- Berdugo, C.A.; Zito, R.; Paulus, S.; Mahlein, A.K. Fusion of sensor data for the detection and differentiation of plant diseases in cucumber. Plant Pathol. 2014, 63, 1344–1356. [Google Scholar] [CrossRef]
- Wang, L.; Poque, S.; Valkonen, J.P. Phenotyping viral infection in sweetpotato using a high-throughput chlorophyll fluorescence and thermal imaging platform. Plant Methods 2019, 15, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schurr, U.; Schuberth, B.; Aloni, R.; Pradel, K.S.; Schmundt, D.; Jahne, B.; Ullrich, C.I. Structural and functional evidence for xylem-mediated water transport and high transpiration in Agrobacterium tumefaciens-induced tumors of Ricinus communis. Bot. Acta 1996, 109, 405–411. [Google Scholar] [CrossRef]
- Boccara, M.; Boue, C.; Garmier, M.; De Paepe, R.; Boccara, A.C. Infra-red thermography revealed a role for mitochondria in pre-symptomatic cooling during harpin-induced hypersensitive response. Plant J. 2001, 28, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Maes, W.H.; Minchin, P.E.H.; Snelgar, W.P.; Steppe, K. Early detection of Psa infection in kiwifruit by means of infrared thermography at leaf and orchard scale. Funct. Plant Biol. 2014, 41, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Spivey, N.W.; Zeng, W.; Liu, P.P.; Fu, Z.Q.; Klessig, D.F.; He, S.Y.; Dong, X. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 2012, 11, 587–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Bueno, M.L.; Pineda, M.; Díaz-Casado, E.; Barón, M. Spatial and temporal dynamics of primary and secondary metabolism in Phaseolus vulgaris challenged by Pseudomonas syringae. Physiol. Plant. 2015, 153, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bueno, M.L.; Granum, E.; Pineda, M.; Flors, V.; Rodríguez-Palenzuela, P.; López-Solanilla, E.; Barón, M. Temporal and spatial resolution of activated plant defense responses in leaves of Nicotiana benthamiana infected with Dickeya dadantii. Front. Plant Sci. 2016, 6, 1209. [Google Scholar] [CrossRef] [Green Version]
- Pineda, M.; Pérez-Bueno, M.L.; Barón, M. Detection of bacterial infection in melon plants by classification methods based on imaging data. Front. Plant Sci. 2018, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Pineda, M.; Luisa Perez-Bueno, M.; Paredes, V.; Baron, M. Use of multicolour fluorescence imaging for diagnosis of bacterial and fungal infection on zucchini by implementing machine learning. Funct. Plant Biol. 2017, 44, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Bueno, M.L.; Pineda, M.; Cabeza, F.; Barón Ayala, M. Multicolor fluorescence imaging as a candidate for disease detection in plant phenotyping. Front. Plant Sci. 2016, 7, 1790. [Google Scholar] [CrossRef] [Green Version]
- Hellebrand, H.J.; Herppich, W.B.; Beuche, H.; Dammer, K.-H.; Linke, M.; Flath, K. Investigations of plant infections by thermal vision and NIR imaging. Int. Agrophys. 2006, 20, 1–10. [Google Scholar]
- Yao, Z.; He, D.; Lei, Y. Thermal imaging for early nondestructive detection of wheat stripe rust. In Proceedings of the 2018 ASABE Annual International Meeting, Detroit, MI, USA, 29 July–1 August 2018; p. 1801728. [Google Scholar]
- Wen, D.-M.; Chen, M.-X.; Zhao, L.; Ji, T.; Li, M.; Yang, X.-T. Use of thermal imaging and Fourier transform infrared spectroscopy for the pre-symptomatic detection of cucumber downy mildew. Eur. J. Plant Pathol. 2019, 155, 405–416. [Google Scholar] [CrossRef]
- Oerke, E.C.; Steiner, U.; Dehne, H.W.; Lindenthal, M. Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. J. Exp. Bot. 2006, 57, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Minaei, S.; Safaie, N. Detection of pre-symptomatic rose powdery-mildew and gray-mold diseases based on thermal vision. Infrared Phys. Technol. 2017, 85, 170–183. [Google Scholar] [CrossRef]
- Raza, S.; Prince, G.; Clarkson, J.P.; Rajpoot, N.M. Automatic detection of diseased tomato plants using thermal and stereo visible light images. PLoS ONE 2015, 10, e0123262. [Google Scholar] [CrossRef]
- Oerke, E.C.; Fröhling, P.; Steiner, U. Thermographic assessment of scab disease on apple leaves. Precis. Agric. 2011, 12, 699–715. [Google Scholar] [CrossRef]
- Belin, É.; Rousseau, D.; Boureau, T.; Caffier, V. Thermography versus chlorophyll fluorescence imaging for detection and quantification of apple scab. Comput. Electron. Agric. 2013, 90, 159–163. [Google Scholar] [CrossRef]
- Sandmann, M.; Grosch, R.; Graefe, J. The use of features from fluorescence, thermography, and NDVI imaging to detect biotic stress in lettuce. Plant Dis. 2018, 102, 1101–1107. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Ling, N.; Dong, X.; Zhu, Y.; Shen, Q.; Guo, S. Thermographic visualization of leaf response in cucumber plants infected with the soil-borne pathogen Fusarium oxysporum f. sp. cucumerinum. Plant Physiol. Biochem. 2012, 61, 153–161. [Google Scholar] [CrossRef]
- Rispail, N.; Rubiales, D. Rapid and efficient estimation of pea resistance to the soil-borne pathogen Fusarium oxysporum by infrared imaging. Sensors 2015, 15, 3988–4000. [Google Scholar] [CrossRef] [Green Version]
- Baranowski, P.; Jedryczka, M.; Mazurek, W.; Babula-Skowronska, D.; Siedliska, A.; Kaczmarek, J. Hyperspectral and thermal imaging of oilseed rape (Brassica napus) response to fungal species of the genus Alternaria. PLoS ONE 2015, 10, e0122913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaerle, L.; Hagenbeek, D.; Vanrobaeys, X.; Van Der Straeten, D. Early detection of nutrient and biotic stress in Phaseolus vulgaris. Int. J. Remote Sens. 2007, 28, 3479–3492. [Google Scholar] [CrossRef]
- Ploetz, R.; Schaffer, B. Effects of flooding and Phytophthora root rot on net gas exchange and growth of avocado. Phytopathology 1989, 79, 204–208. [Google Scholar] [CrossRef]
- Granum, E.; Pérez-Bueno, M.L.; Calderón, C.E.; Ramos, C.; de Vicente, A.; Cazorla, F.M.; Barón, M. Metabolic responses of avocado plants to stress induced by Rosellinia necatrix analysed by fluorescence and thermal imaging. Eur. J. Plant Pathol. 2015, 142, 625–632. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Vida, C.; Fernández-Ortuño, D.; Torés, J.A.; de Vicente, A.; Cazorla, F.M.; Barón, M. Detection of white root rot in avocado trees by remote sensing. Plant Dis. 2019, 103, 1119–1125. [Google Scholar] [CrossRef]
- Aldea, M.; Hamilton, J.G.; Resti, J.P.; Zangerl, A.R.; Berenbaum, M.R.; DeLucia, E.H. Indirect effects of insect herbivory on leaf gas exchange in soybean. Plant Cell Environ. 2005, 28, 402–411. [Google Scholar] [CrossRef]
- Tang, J.Y.; Zielinski, R.E.; Zangerl, A.R.; Crofts, A.R.; Berenbaum, M.R.; DeLucia, E.H. The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera: Noctuidae) on photosynthesis in Arabidopsis thaliana. J. Exp. Bot. 2006, 57, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Nabity, P.D.; Zavala, J.A.; DeLucia, E.H. Herbivore induction of jasmonic acid and chemical defences reduce photosynthesis in Nicotiana attenuata. J. Exp. Bot. 2013, 64, 685–694. [Google Scholar] [CrossRef] [Green Version]
- Nabity, P.D.; Hillstrom, M.L.; Lindroth, R.L.; DeLucia, E.H. Elevated CO2 interacts with herbivory to alter chlorophyll fluorescence and leaf temperature in Betula papyrifera and Populus tremuloides. Oecologia 2012, 169, 905–913. [Google Scholar] [CrossRef]
- Joalland, S.; Screpanti, C.; Liebisch, F.; Varella, H.V.; Gaume, A.; Walter, A. Comparison of visible imaging, thermography and spectrometry methods to evaluate the effect of Heterodera schachtii inoculation on sugar beets. Plant Methods 2017, 13, 14. [Google Scholar] [CrossRef]
- Ortiz-Bustos, C.M.; Pérez-Bueno, M.L.; Barón, M.; Molinero-Ruiz, L. Use of blue-green fluorescence and thermal imaging in the early detection of sunflower infection by the root parasitic weed Orobanche cumana Wallr. Front. Plant Sci. 2017, 8, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Schmitz, A.; Kiewnick, S.; Schlang, J.; Sikora, R.A. Use of high resolution digital thermography to detect Heterodera schachtii infestation in sugar beets. Commun. Agric. Appl. Biol. Sci. 2004, 69, 359–363. [Google Scholar] [PubMed]
- Wang, Y.; Zia-Khan, S.; Owusu-Adu, S.; Miedaner, T.; Müller, J. Early detection of Zymoseptoria tritici in winter wheat by infrared thermography. Agriculture 2019, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, K.W. Outdoor infrared imaging for spatial and temporal thermography: A case study of necrotic versus healthy leaf areas on woody plants. J. Phytopathol. 2020. [Google Scholar] [CrossRef]
- Aldea, M.; Hamilton, J.G.; Resti, J.P.; Zangerl, A.R.; Berenbaum, M.R.; Frank, T.D.; Delucia, E.H. Comparison of photosynthetic damage from arthropod herbivory and pathogen infection in understory hardwood saplings. Oecologia 2006, 149, 221–232. [Google Scholar] [CrossRef]
- Sankaran, S.; Maja, J.M.; Buchanon, S.; Ehsani, R. Huanglongbing (citrus greening) detection using visible, near infrared and thermal imaging techniques. Sensors 2013, 13, 2117–2130. [Google Scholar] [CrossRef] [Green Version]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.A.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M.; et al. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [CrossRef]
- López-López, M.; Calderón, R.; González-Dugo, V.; Zarco-Tejada, P.; Fereres, E. Early detection and quantification of almond red leaf blotch using high-resolution hyperspectral and thermal imagery. Remote Sens. 2016, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Omran, E.-S.E. Early sensing of peanut leaf spot using spectroscopy and thermal imaging. Arch. Agron. Soil Sci. 2017, 63, 883–896. [Google Scholar] [CrossRef]
- Calderón, R.; Montes-Borrego, M.; Landa, B.; Navas-Cortés, J.; Zarco-Tejada, P. Detection of downy mildew of opium poppy using high-resolution multi-spectral and thermal imagery acquired with an unmanned aerial vehicle. Precis. Agric. 2014, 15, 639–661. [Google Scholar] [CrossRef]
- Loladze, A.; Rodrigues, F.A.; Toledo, F.; San Vicente, F.; Gerard, B.; Boddupalli, M.P. Application of remote sensing for phenotyping tar spot complex resistance in maize. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.Y.; Chen, W.D.; Sankaran, S. High-throughput field phenotyping of Ascochyta blight disease severity in chickpea. Crop Protect. 2019, 125, 11. [Google Scholar] [CrossRef]
- Calderón, R.; Navas-Cortés, J.A.; Zarco-Tejada, P.J. Early detection and quantification of Verticillium wilt in olive using hyperspectral and thermal imagery over large areas. Remote Sens. Environ. 2015, 7, 5584–5610. [Google Scholar] [CrossRef] [Green Version]
- Calderón, R.; Navas-Cortés, J.A.; Lucena, C.; Zarco-Tejada, P.J. High-resolution airborne hyperspectral and thermal imagery for early, detection of Verticillium wilt of olive using fluorescence, temperature and narrow-band spectral indices. Remote Sens. Environ. 2013, 139, 231–245. [Google Scholar] [CrossRef]
- Kwon, T.-r.; Kim, K.-h.; Yoon, H.-J.; Lee, S.-k.; Kim, B.-k.; Siddiqui, Z.S. Phenotyping of plants for drought and salt tolerance using infra-red thermography. Plant Breed. Biotechnol. 2015, 3, 299–307. [Google Scholar] [CrossRef] [Green Version]
- James, R.A.; Sirault, X.R.R. Infrared thermography in plant phenotyping for salinity tolerance. In Plant Salt Tolerance: Methods and Protocols; Shabala, S., Cuin, T.A., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 173–189. [Google Scholar] [CrossRef]
- Kim, S.L.; Kim, N.; Lee, H.; Lee, E.; Cheon, K.-S.; Kim, M.; Baek, J.; Choi, I.; Ji, H.; Yoon, I.S.; et al. High-throughput phenotyping platform for analyzing drought tolerance in rice. Planta 2020, 252, 38. [Google Scholar] [CrossRef]
- Biju, S.; Fuentes, S.; Gupta, D. The use of infrared thermal imaging as a non-destructive screening tool for identifying drought-tolerant lentil genotypes. Plant Physiol. Biochem. 2018, 127, 11–24. [Google Scholar] [CrossRef]
- Pan, R.; Jiang, W.; Wang, Q.; Xu, L.; Shabala, S.; Zhang, W.Y. Differential response of growth and photosynthesis in diverse cotton genotypes under hypoxia stress. Photosynthetica 2019, 57, 772–779. [Google Scholar] [CrossRef] [Green Version]
- Vollsnes, A.V.; Eriksen, A.B.; Otterholt, E.; Kvaal, K.; Oxaal, U.; Futsaether, C.M. Visible foliar injury and infrared imaging show that daylength affects short-term recovery after ozone stress in Trifolium subterraneum. J. Exp. Bot. 2009, 60, 3677–3686. [Google Scholar] [CrossRef]
- Rippa, M.; Ambrosone, A.; Leone, A.; Mormile, P. Active thermography for real time monitoring of UV-B plant interactions. J. Photochem. Photobiol. B Biol. 2020, 208, 111900. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.P.; Wisniewski, M. The use of infrared thermal imaging in the study of ice nucleation and freezing of plants. J. Therm. Biol. 1998, 23, 81–89. [Google Scholar] [CrossRef]
- Wisniewski, M.; Glenn, D.M.; Fuller, M.P. Use of a hydrophobic particle film as a barrier to extrinsic ice nucleation in tomato plants. J. Am. Soc. Hort. Sci. 2002, 127, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Workmaster, B.A.A.; Palta, J.P.; Wisniewski, M. Ice nucleation and propagation in cranberry uprights and fruit using infrared video thermography. J. Am. Soc. Hort. Sci. 1999, 124, 619–625. [Google Scholar] [CrossRef]
- Carter, J.; Brennan, R.; Wisniewski, M. Patterns of ice formation and movement in blackcurrant. HortScience 2001, 36, 1027–1032. [Google Scholar] [CrossRef] [Green Version]
- Gusta, L.V.; Wisniewski, M.; Nesbitt, N.T.; Gusta, M.L. The effect of water, sugars, and proteins on the pattern of ice nucleation and propagation in acclimated and nonacclimated canola leaves. Plant Physiol. 2004, 135, 1642–1653. [Google Scholar] [CrossRef] [Green Version]
- Pearce, R.S.; Fuller, M.P. Freezing of barley studied by infrared video thermography. Plant Physiol. 2001, 125, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Stegner, M.; Schäfernolte, T.; Neuner, G. New insights in potato leaf freezing by infrared thermography. Appl. Sci. 2019, 9, 819. [Google Scholar] [CrossRef] [Green Version]
- Chaerle, L.; Hulsen, K.; Hermans, C.; Strasser, R.J.; Valcke, R.; Hofte, M.; Van der Straeten, D. Robotized time-lapse imaging to assess in-planta uptake of phenylurea herbicides and their microbial degradation. Physiol. Plant. 2003, 118, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Takayama, K.; Omasa, K. Early detection of photosynthetic dysfunction caused by a herbicide (Basta) using chlorophyll fluorescence and thermal imaging system. J. Agric. Meteorol. 2005, 60, 1179–1181. [Google Scholar] [CrossRef]
- Vítek, P.; Veselá, B.; Klem, K. Spatial and temporal variability of plant leaf responses cascade after PSII inhibition: Raman, chlorophyll fluorescence and infrared thermal imaging. Sensors 2020, 20, 1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellvert, J.; Marsal, J.; Girona, J.; Zarco-Tejada, P. Seasonal evolution of crop water stress index in grapevine varieties determined with high-resolution remote sensing thermal imagery. Irrig. Sci. 2014, 33, 81–93. [Google Scholar] [CrossRef]
- González-Dugo, M.P.; González-Piqueras, J.; Campos, I.; Andréu, A.; Balbontín, C.; Calera, A. Evapotranspiration monitoring in a vineyard using satellite-based thermal remote sensing. In Proceedings of the Remote Sensing for Agriculture, Ecosystems, and Hydrology XIV, Edinburgh, UK, 24–26 September 2012. [Google Scholar]
- Suárez, L.; Zarco-Tejada, P.J.; Sepulcre-Cantó, G.; Pérez-Priego, O.; Miller, J.R.; Jiménez-Muñoz, J.C.; Sobrino, J. Assessing canopy PRI for water stress detection with diurnal airborne imagery. Remote Sens. Environ. 2008, 112, 560–575. [Google Scholar] [CrossRef]
- Sepulcre-Cantó, G.; Zarco-Tejada, P.J.; Sobrino, J.A.; Jiménez-Berni, J.A.; Jiménez-Muñoz, J.C.; Gastellu-Etchegorry, J.P. Discriminating irrigated and rainfed olive orchards with thermal ASTER imagery and DART 3D simulation. Agric. For. Meteorol. 2009, 149, 962–975. [Google Scholar] [CrossRef] [Green Version]
- Casari, R.A.C.N.; Paiva, D.S.; Silva, V.N.B.; Ferreira, T.M.M.; Souza, M.T.; Oliveira, N.G.; Kobayashi, A.K.; Molinari, H.B.C.; Santos, T.T.; Gomide, R.L.; et al. Using thermography to confirm genotypic variation for drought response in maize. Int. J. Mol. Sci. 2019, 20, 2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rischbeck, P.; Cardellach, P.; Mistele, B.; Schmidhalter, U. Thermal phenotyping of stomatal sensitivity in spring barley. J. Agron. Crop Sci. 2017, 203, 483–493. [Google Scholar] [CrossRef]
- Romero-Bravo, S.; Mendez-Espinoza, A.M.; Garriga, M.; Estrada, F.; Escobar, A.; Gonzalez-Martinez, L.; Poblete-Echeverria, C.; Sepulveda, D.; Matus, I.; Castillo, D.; et al. Thermal imaging reliability for estimating grain yield and carbon isotope discrimination in wheat genotypes: Importance of the environmental conditions. Sensors 2019, 19, 2676. [Google Scholar] [CrossRef] [Green Version]
- Gracia-Romero, A.; Kefauver, S.C.; Fernández-Gallego, J.A.; Vergara-Diaz, O.; Nieto-Taladriz, M.T.; Araus, J.L. UAV and ground image-based phenotyping: A proof of concept with durum wheat. Remote Sens. 2019, 11, 1244. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Virosta, Á.; Sánchez-Gómez, D. Thermography as a tool to assess inter-cultivar variability in garlic performance along variations of soil water availability. Remote Sens. 2020, 12, 2990. [Google Scholar] [CrossRef]
- Tilling, A.K.; O’Leary, G.J.; Ferwerda, J.G.; Jones, S.D.; Fitzgerald, G.J.; Rodriguez, D.; Belford, R. Remote sensing of nitrogen and water stress in wheat. Field Crop. Res. 2007, 104, 77–85. [Google Scholar] [CrossRef]
- Kefauver, S.C.; Vicente, R.; Vergara-Díaz, O.; Fernández-Gallego, J.A.; Kerfal, S.; López, A.; Melichar, J.P.E.; Molins, M.D.S.; Araus, J.L. Comparative UAV and field phenotyping to assess yield and nitrogen use efficiency in hybrid and conventional barley. Front. Plant Sci. 2017, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Pramsohler, M.; Hacker, J.; Neuner, G. Freezing pattern and frost killing temperature of apple (Malus domestica) wood under controlled conditions and in nature. Tree Physiol. 2012, 32, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangha, H.S.; Sharda, A.; Koch, L.; Prabhakar, P.; Wang, G. Impact of camera focal length and sUAS flying altitude on spatial crop canopy temperature evaluation. Comput. Electron. Agric. 2020, 172, 105344. [Google Scholar] [CrossRef]
- Furbank, R.T.; Jiménez-Berni, J.A.; George-Jaeggli, B.; Potgieter, A.B.; Deery, D.M. Field crop phenomics: Enabling breeding for radiation use efficiency and biomass in cereal crops. New Phytol. 2019, 223, 1714–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppens, F.; Wuyts, N.; Inzé, D.; Dhondt, S. Unlocking the potential of plant phenotyping data through integration and data-driven approaches. Curr. Opin. Syst. Biol. 2017, 4, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z.; Li, J.; Zhang, X.; Wang, R. A non-invasive analysis of seed vigor by infrared thermography. Plants 2020, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Dakhiya, Y.; Green, R.M. Thermal imaging as a noninvasive technique for analyzing circadian rhythms in plants. New Phytol. 2019, 224, 1685–1696. [Google Scholar] [CrossRef]
- Lei, L. Imaging plant rhythms. Nat. Plants 2019, 5, 911. [Google Scholar] [CrossRef]
- Kitaya, Y. Plant Factory and Space Development, “Space Farm”; Elsevier Science Bv: Amsterdam, The Netherlands, 2019; pp. 363–379. [Google Scholar] [CrossRef]
- Govindasamy, V.; George, P.; Aher, L.; Ramesh, S.V.; Thangasamy, A.; Anandan, S.; Raina, S.K.; Kumar, M.; Rane, J.; Annapurna, K.; et al. Comparative conventional and phenomics approaches to assess symbiotic effectiveness of Bradyrhizobia strains in soybean (Glycine max L. Merrill) to drought. Sci. Rep. 2017, 7, 14. [Google Scholar] [CrossRef]
- Jiang, W.; Pan, R.; Wu, C.; Xu, L.; Abdelaziz, M.E.; Oelmüller, R.; Zhang, W. Piriformospora indica enhances freezing tolerance and post-thaw recovery in Arabidopsis by stimulating the expression of CBF genes. Plant Signal. Behav. 2020, 15, 1745472. [Google Scholar] [CrossRef]
- Tsai, H.-J.; Shao, K.-H.; Chan, M.-T.; Cheng, C.-P.; Yeh, K.-W.; Oelmüller, R.; Wang, S.-J. Piriformospora indica symbiosis improves water stress tolerance of rice through regulating stomata behavior and ROS scavenging systems. Plant Signal. Behav. 2020, 15, 1722447. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A.; Hassen, A.I.; Labuschagne, N. Rhizobacteria-induced systemic resilience in Sorghum bicolor (L.) moench against Fusarium pseudograminearum crown rot under drought stress conditions. Biol. Control 2020, 151, 104395. [Google Scholar] [CrossRef]
- Vadivambal, R.; Jayas, D. Applications of thermal imaging in agriculture and food industry—A review. Food Bioprocess Technol. 2011, 4, 186–199. [Google Scholar] [CrossRef]
- Negi, J.; Hashimoto-Sugimoto, M.; Kusumi, K.; Iba, K. New approaches to the biology of stomatal guard cells. Plant Cell Physiol. 2014, 55, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.B.; Holroyd, G.; Hetherington, A.M.; Ng, C.K.Y. Seeing ‘cool’ and ‘hot’-infrared thermography as a tool for non-invasive, high-throughput screening of Arabidopsis guard cell signalling mutants. J. Exp. Bot. 2004, 55, 1187–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Category | Stress Factor | Application | Plant | Effect on Leaf Temperature | Ref. |
|---|---|---|---|---|---|
| Water | Drought | Roots | Papaya, spinach, fava bean, corn, wheat, rice, lentil | ↑ detected as alterations in TIR stress indices | [28,44,45,60,61,143,144] |
| Salinity | Roots | Barley, rice, wheat | ↑ proportional to salt concentration | [66,67] | |
| Nutrient deficiency | O2 | Roots | Cotton | ↑ stomatal closure | [145] |
| Mg | Roots | Bean | ↑ prior to chlorosis | [117] | |
| Atmosph. | High O3 | Leaves | Subterranean clover | ↑ under long-day conditions | [146] |
| UV-B | Leaves | Pothos plant, Arabidopsis | ↑ under UV-B light | [147] | |
| Low T | Ice nucleation | Potato, cranberry, oilseed rape, barley blackcurrant, tomato | ↑ since ice nucleation is an exothermic process | [148,149,150,151,152,153,154] | |
| Herbicide | Linuron | Roots | Bean | ↑ from vascular tissues towards the leaf edges | [155] |
| Glufosinate | Leaves (spray) | Bean | ↑ gradually | [156] | |
| Metribuzin | Leaves (droplets) | Goosefoot | ↑ in the spot, expanding to the rest of the leaf | [157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda, M.; Barón, M.; Pérez-Bueno, M.-L. Thermal Imaging for Plant Stress Detection and Phenotyping. Remote Sens. 2021, 13, 68. https://doi.org/10.3390/rs13010068
Pineda M, Barón M, Pérez-Bueno M-L. Thermal Imaging for Plant Stress Detection and Phenotyping. Remote Sensing. 2021; 13(1):68. https://doi.org/10.3390/rs13010068
Chicago/Turabian StylePineda, Mónica, Matilde Barón, and María-Luisa Pérez-Bueno. 2021. "Thermal Imaging for Plant Stress Detection and Phenotyping" Remote Sensing 13, no. 1: 68. https://doi.org/10.3390/rs13010068
APA StylePineda, M., Barón, M., & Pérez-Bueno, M. -L. (2021). Thermal Imaging for Plant Stress Detection and Phenotyping. Remote Sensing, 13(1), 68. https://doi.org/10.3390/rs13010068