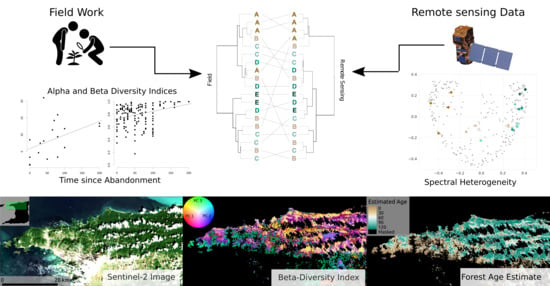
A Remote Sensing Approach to Understanding Patterns of Secondary Succession in Tropical Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Field Plot Network
2.2. Survey Methods
2.3. Satellite Imaging Surveys
2.4. Diversity Indices Computed from Ground Inventories
2.4.1. Alpha-Diversity
2.4.2. Beta-Diversity
2.4.3. Remote Sensing Analyses
2.5. Comparison between Ground Observations and Remotely Sensed Information
2.5.1. Spatial Sampling of Satellite Images
2.5.2. Alpha and Beta-Diversity Analyses
2.5.3. Mapping Forest Age
3. Results
3.1. Alpha Diversity
3.2. Beta Diversity
3.2.1. Bray-Curtis Dissimilarity
3.2.2. Ordination of Bray Curtis Dissimilarity Computed from Sentinel-2
3.3. Mapping Forest Age
4. Discussion
4.1. Alpha and Beta-Diversity Comparison
4.2. Mapping Forest Age
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Blowes, S.A.; Supp, S.R.; Antão, L.H.; Bates, A.; Bruelheide, H.; Chase, J.M.; Moyes, F.; Magurran, A.; McGill, B.; Myers-Smith, I.H.; et al. The Geography of Biodiversity Change in Marine and Terrestrial Assemblages. Science 2019, 366, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Isbell, F.; Gonzalez, A.; Loreau, M.; Cowles, J.; Díaz, S.; Hector, A.; Mace, G.M.; Wardle, D.A.; O’Connor, M.I.; Duffy, J.E.; et al. Linking the Influence and Dependence of People on Biodiversity across Scales. Nature 2017, 546, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Magurran, A.E.; Dornelas, M. Biological Diversity in a Changing World. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3593–3597. [Google Scholar] [CrossRef]
- Pereira, H.M.; Rosa, I.M.D.; Martins, I.S.; Kim, H.; Leadley, P.; Popp, A.; van Vuuren, D.P.; Hurtt, G.; Anthoni, P.; Arneth, A.; et al. Global Trends in Biodiversity and Ecosystem Services from 1900 to 2050. Biorvix 2020. [Google Scholar] [CrossRef]
- Diamond, J.M. “Normal” extinctions of isolated populations. In Extinctions; University of Chicago Press: Chicago, IL, USA, 1984; pp. 191–246. [Google Scholar]
- Ehrlich, P.R.; Pringle, R.M. Where Does Biodiversity Go from Here? A Grim Business-as-Usual Forecast and a Hopeful Portfolio of Partial Solutions. Proc. Natl. Acad. Sci. USA 2008, 105, 11579–11586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semper-Pascual, A.; Decarre, J.; Baumann, M.; Busso, J.M.; Camino, M.; Gómez-Valencia, B.; Kuemmerle, T. Biodiversity Loss in Deforestation Frontiers: Linking Occupancy Modelling and Physiological Stress Indicators to Understand Local Extinctions. Biol. Conserv. 2019, 236, 281–288. [Google Scholar] [CrossRef]
- Dornelas, M.; Gotelli, N.J.; McGill, B.; Shimadzu, H.; Moyes, F.; Sievers, C.; Magurran, A.E. Assemblage Time Series Reveal Biodiversity Change but Not Systematic Loss. Science 2014, 344, 296–299. [Google Scholar] [CrossRef] [Green Version]
- McGill, B.J.; Dornelas, M.; Gotelli, N.J.; Magurran, A.E. Fifteen Forms of Biodiversity Trend in the Anthropocene. Trends Ecol. Evol. 2015, 30, 104–113. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global Effects of Land Use on Local Terrestrial Biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity Loss and Its Impact on Humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- The Strategic Plan for Biodiversity 2011–2020 and the Aichi Biodiversity Targets. Available online: https://www.cbd.int/sp/ (accessed on 1 March 2021).
- Gaston, K.J. Global Patterns in Biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Honnay, O. Forest Restoration, Biodiversity and Ecosystem Functioning. BMC Ecol. 2011, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Betts, M.G.; Wolf, C.; Ripple, W.J.; Phalan, B.; Millers, K.A.; Duarte, A.; Butchart, S.H.M.; Levi, T. Global Forest Loss Disproportionately Erodes Biodiversity in Intact Landscapes. Nature 2017, 547, 441–444. [Google Scholar] [CrossRef]
- Morales-Hidalgo, D.; Oswalt, S.N.; Somanathan, E. Status and Trends in Global Primary Forest, Protected Areas, and Areas Designated for Conservation of Biodiversity from the Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Myers, N. The Conversion of Tropical Forests. Environ. Sci. Policy Sustain. Dev. 1980, 22, 6–13. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Shvidenko, A.; McCallum, I.; Nilsson, S. Forest and woodlands systems. In Ecosystems and Human Well-Being: Current State and Trends; Hassan, R., Scholes, R., Ash, N., Eds.; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Global Forest Watch Tree Cover Loss and Gain (Global). Available online: www.globalforestwatch.org (accessed on 20 March 2021).
- Arroyo-Rodríguez, V.; Melo, F.P.L.; Martínez-Ramos, M.; Bongers, F.; Chazdon, R.L.; Meave, J.A.; Norden, N.; Santos, B.A.; Leal, I.R.; Tabarelli, M. Multiple Successional Pathways in Human-Modified Tropical Landscapes: New Insights from Forest Succession, Forest Fragmentation and Landscape Ecology Research. Biol. Rev. 2017, 92, 326–340. [Google Scholar] [CrossRef]
- Derroire, G.; Balvanera, P.; Castellanos-Castro, C.; Decocq, G.; Kennard, D.K.; Lebrija-Trejos, E.; Leiva, J.A.; Odén, P.-C.; Powers, J.S.; Rico-Gray, V.; et al. Resilience of Tropical Dry Forests—A Meta-Analysis of Changes in Species Diversity and Composition during Secondary Succession. Oikos 2016, 125, 1386–1397. [Google Scholar] [CrossRef] [Green Version]
- Lebrija-Trejos, E.; Bongers, F.; Pérez-García, E.A.; Meave, J.A. Successional Change and Resilience of a Very Dry Tropical Deciduous Forest Following Shifting Agriculture. Biotropica 2008, 40, 422–431. [Google Scholar] [CrossRef]
- Martin, P.A.; Newton, A.C.; Bullock, J.M. Carbon Pools Recover More Quickly than Plant Biodiversity in Tropical Secondary Forests. Proc. R. Soc. B Biol. Sci. 2013, 280, 20132236. [Google Scholar] [CrossRef] [PubMed]
- Bernier, P.Y.; Paré, D.; Stinson, G.; Bridge, S.R.J.; Kishchuk, B.E.; Lemprière, T.C.; Thiffault, E.; Titus, B.D.; Vasbinder, W. Moving beyond the Concept of “Primary Forest” as a Metric of Forest Environment Quality. Ecol. Appl. 2017, 27, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Rozendaal, D.M.A.; Bongers, F.; Aide, T.M.; Alvarez-Dávila, E.; Ascarrunz, N.; Balvanera, P.; Becknell, J.M.; Bentos, T.V.; Brancalion, P.H.S.; Cabral, G.A.L.; et al. Biodiversity Recovery of Neotropical Secondary Forests. Sci. Adv. 2019, 5, eaau3114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levrel, H.; Fontaine, B.; Henry, P.-Y.; Jiguet, F.; Julliard, R.; Kerbiriou, C.; Couvet, D. Balancing State and Volunteer Investment in Biodiversity Monitoring for the Implementation of CBD Indicators: A French Example. Ecol. Econ. 2010, 69, 1580–1586. [Google Scholar] [CrossRef] [Green Version]
- Schiller, A.; Hunsaker, C.T.; Kane, M.A.; Wolfe, A.K.; Dale, V.H.; Suter, G.W.; Russell, C.S.; Pion, G.; Jensen, M.H.; Konar, V.C. Communicating Ecological Indicators to Decision Makers and the Public. Conserv. Ecol. 2001, 5, art19. [Google Scholar] [CrossRef]
- Barton, P.S.; Cunningham, S.A.; Manning, A.D.; Gibb, H.; Lindenmayer, D.B.; Didham, R.K. The Spatial Scaling of Beta Diversity: Spatial Scaling of Beta Diversity. Glob. Ecol. Biogeogr. 2013, 22, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, B.J.; Gonzalez, A.; Allington, G.R.H.; Loreau, M. Is Local Biodiversity Declining or Not? A Summary of the Debate over Analysis of Species Richness Time Trends. Biol. Conserv. 2018, 219, 175–183. [Google Scholar] [CrossRef]
- Proença, V.; Martin, L.J.; Pereira, H.M.; Fernandez, M.; McRae, L.; Belnap, J.; Böhm, M.; Brummitt, N.; García-Moreno, J.; Gregory, R.D.; et al. Global Biodiversity Monitoring: From Data Sources to Essential Biodiversity Variables. Biol. Conserv. 2017, 213, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.B. Biodiversity Monitoring, Earth Observations and the Ecology of Scale. Ecol. Lett. 2018, 21, 1572–1585. [Google Scholar] [CrossRef]
- Luque, S.; Pettorelli, N.; Vihervaara, P.; Wegmann, M. Improving Biodiversity Monitoring Using Satellite Remote Sensing to Provide Solutions towards the 2020 Conservation Targets. Methods Ecol. Evol. 2018, 9, 1784–1786. [Google Scholar] [CrossRef] [Green Version]
- Mulatu, K.; Mora, B.; Kooistra, L.; Herold, M. Biodiversity Monitoring in Changing Tropical Forests: A Review of Approaches and New Opportunities. Remote Sens. 2017, 9, 1059. [Google Scholar] [CrossRef] [Green Version]
- Rocchini, D.; Salvatori, N.; Beierkuhnlein, C.; Chiarucci, A.; de Boissieu, F.; Förster, M.; Garzon-Lopez, C.X.; Gillespie, T.W.; Hauffe, H.C.; He, K.S.; et al. From Local Spectral Species to Global Spectral Communities: A Benchmark for Ecosystem Diversity Estimate by Remote Sensing. Ecol. Inform. 2021, 61, 101195. [Google Scholar] [CrossRef]
- Wang, R.; Gamon, J.A. Remote Sensing of Terrestrial Plant Biodiversity. Remote Sens. Environ. 2019, 231, 111218. [Google Scholar] [CrossRef]
- Rocchini, D.; Boyd, D.S.; Féret, J.-B.; Foody, G.M.; He, K.S.; Lausch, A.; Nagendra, H.; Wegmann, M.; Pettorelli, N. Satellite Remote Sensing to Monitor Species Diversity: Potential and Pitfalls. Remote Sens. Ecol. Conserv. 2016, 2, 25–36. [Google Scholar] [CrossRef]
- Baldeck, C.A.; Asner, G.P.; Martin, R.E.; Anderson, C.B.; Knapp, D.E.; Kellner, J.R.; Wright, S.J. Operational Tree Species Mapping in a Diverse Tropical Forest with Airborne Imaging Spectroscopy. PLoS ONE 2015, 10, e0118403. [Google Scholar] [CrossRef]
- Clark, M.; Roberts, D.; Clark, D. Hyperspectral Discrimination of Tropical Rain Forest Tree Species at Leaf to Crown Scales. Remote Sens. Environ. 2005, 96, 375–398. [Google Scholar] [CrossRef]
- Féret, J.-B.; Asner, G.P. Tree Species Discrimination in Tropical Forests Using Airborne Imaging Spectroscopy. IEEE Trans. Geosci. Remote Sens. 2013, 51, 73–84. [Google Scholar] [CrossRef]
- Draper, F.C.; Baraloto, C.; Brodrick, P.G.; Phillips, O.L.; Martinez, R.V.; Honorio Coronado, E.N.; Baker, T.R.; Zárate Gómez, R.; Amasifuen Guerra, C.A.; Flores, M.; et al. Imaging Spectroscopy Predicts Variable Distance Decay across Contrasting Amazonian Tree Communities. J. Ecol. 2019, 107, 696–710. [Google Scholar] [CrossRef]
- Vaglio Laurin, G.; Puletti, N.; Hawthorne, W.; Liesenberg, V.; Corona, P.; Papale, D.; Chen, Q.; Valentini, R. Discrimination of Tropical Forest Types, Dominant Species, and Mapping of Functional Guilds by Hyperspectral and Simulated Multispectral Sentinel-2 Data. Remote Sens. Environ. 2016, 176, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Schneider, F.D.; Morsdorf, F.; Schmid, B.; Petchey, O.L.; Hueni, A.; Schimel, D.S.; Schaepman, M.E. Mapping Functional Diversity from Remotely Sensed Morphological and Physiological Forest Traits. Nat. Commun. 2017, 8, 1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asner, G.P.; Martin, R.E.; Knapp, D.E.; Tupayachi, R.; Anderson, C.B.; Sinca, F.; Vaughn, N.R.; Llactayo, W. Airborne Laser-Guided Imaging Spectroscopy to Map Forest Trait Diversity and Guide Conservation. Science 2017, 355, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Durán, S.M.; Martin, R.E.; Díaz, S.; Maitner, B.S.; Malhi, Y.; Salinas, N.; Shenkin, A.; Silman, M.R.; Wieczynski, D.J.; Asner, G.P.; et al. Informing Trait-Based Ecology by Assessing Remotely Sensed Functional Diversity across a Broad Tropical Temperature Gradient. Sci. Adv. 2019, 5, eaaw8114. [Google Scholar] [CrossRef] [Green Version]
- Carreiras, J.M.B.; Jones, J.; Lucas, R.M.; Shimabukuro, Y.E. Mapping Major Land Cover Types and Retrieving the Age of Secondary Forests in the Brazilian Amazon by Combining Single-Date Optical and Radar Remote Sensing Data. Remote Sens. Environ. 2017, 194, 16–32. [Google Scholar] [CrossRef] [Green Version]
- Fujiki, S.; Aoyagi, R.; Tanaka, A.; Imai, N.; Kusma, A.D.; Kurniawan, Y.; Lee, Y.F.; Sugau, J.B.; Pereira, J.T.; Samejima, H.; et al. Large-Scale Mapping of Tree-Community Composition as a Surrogate of Forest Degradation in Bornean Tropical Rain Forests. Land 2016, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Palmer, M.W.; Earls, P.G.; Hoagland, B.W.; White, P.S.; Wohlgemuth, T. Quantitative Tools for Perfecting Species Lists. Environmetrics 2002, 13, 121–137. [Google Scholar] [CrossRef]
- Schweiger, A.K.; Cavender-Bares, J.; Townsend, P.A.; Hobbie, S.E.; Madritch, M.D.; Wang, R.; Tilman, D.; Gamon, J.A. Plant Spectral Diversity Integrates Functional and Phylogenetic Components of Biodiversity and Predicts Ecosystem Function. Nat. Ecol. Evol. 2018, 2, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J.; Gamon, J.A.; Hobbie, S.E.; Madritch, M.D.; Meireles, J.E.; Schweiger, A.K.; Townsend, P.A. Harnessing Plant Spectra to Integrate the Biodiversity Sciences across Biological and Spatial Scales. Am. J. Bot. 2017, 104, 966–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laliberté, E.; Schweiger, A.K.; Legendre, P. Partitioning Plant Spectral Diversity into Alpha and Beta Components. Ecol. Lett. 2020, 23, 370–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Féret, J.-B.; Asner, G.P. Mapping Tropical Forest Canopy Diversity Using High-fidelity Imaging Spectroscopy. Ecol. Appl. Publ. Ecol. Soc. Am. 2014, 24, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Rocchini, D.; Balkenhol, N.; Carter, G.A.; Foody, G.M.; Gillespie, T.W.; He, K.S.; Kark, S.; Levin, N.; Lucas, K.; Luoto, M.; et al. Remotely Sensed Spectral Heterogeneity as a Proxy of Species Diversity: Recent Advances and Open Challenges. Ecol. Inform. 2010, 5, 318–329. [Google Scholar] [CrossRef]
- Schäfer, E.; Heiskanen, J.; Heikinheimo, V.; Pellikka, P. Mapping Tree Species Diversity of a Tropical Montane Forest by Unsupervised Clustering of Airborne Imaging Spectroscopy Data. Ecol. Indic. 2016, 64, 49–58. [Google Scholar] [CrossRef]
- Rocchini, D.; Luque, S.; Pettorelli, N.; Bastin, L.; Doktor, D.; Faedi, N.; Feilhauer, H.; Féret, J.-B.; Foody, G.M.; Gavish, Y.; et al. Measuring β-Diversity by Remote Sensing: A Challenge for Biodiversity Monitoring. Methods Ecol. Evol. 2018, 9, 1787–1798. [Google Scholar] [CrossRef] [Green Version]
- Torresani, M.; Rocchini, D.; Sonnenschein, R.; Zebisch, M.; Hauffe, H.C.; Heym, M.; Pretzsch, H.; Tonon, G. Height Variation Hypothesis: A New Approach for Estimating Forest Species Diversity with CHM LiDAR Data. Ecol. Indic. 2020, 117, 106520. [Google Scholar] [CrossRef]
- Chazdon, R.L. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation; University of Chicago Press: Chicago, IL, USA, 2014; ISBN 978-0-226-11807-9. [Google Scholar]
- Egler, F.E. Vegetation Science Concepts I. Initial Floristic Composition, a Factor in Old-Field Vegetation Development with 2 Figs. Veg. Acta Geobot. 1954, 4, 412–417. [Google Scholar] [CrossRef]
- Finegan, B. Pattern and Process in Neotropical Secondary Rain Forests: The First 100 Years of Succession. Trends Ecol. Evol. 1996, 11, 119–124. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Ostertag, R. Neotropical Secondary Forest Succession: Changes in Structural and Functional Characteristics. For. Ecol. Manag. 2001, 148, 185–206. [Google Scholar] [CrossRef]
- Bekele, F. The History of Cocoa Production in Trinidad and Tobago. In Proceedings of the APASTT Seminar, Re-Vitalisation of the Trinidad & Tobago Cocoa Industry, St. Augustine, FL, USA, 20 September 2003; pp. 4–12. [Google Scholar]
- NATT. Honouring Our Industrial Roots: Sugar, Cocoa, Asphalt and Oil; National Archives of Trinidad and Tobago: Port of Spain, Trinidad, 2018. [Google Scholar]
- Kenefick, M.; Restall, R.L.; Hayes, F.E. Birds of Trinidad & Tobago; Bloomsbury Publishing PLC: London, UK, 2011; ISBN 978-1-4729-4152-7. [Google Scholar]
- Arnold, H.; Deacon, A.E.; Hulme, M.F.; Sansom, A.; Jaggernauth, D.; Magurran, A.E. Contrasting Trends in Biodiversity of Birds and Trees during Succession Following Cacao Agroforest Abandonment. J. Appl. Ecol. 2021. [Google Scholar] [CrossRef]
- QGIS Geographic Information System. Open Source Geospatial Foundation. Available online: http://qgis.org (accessed on 1 March 2021).
- ESA SP. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services; Fletcher, K., European Space Agency, Eds.; ESA Communications: Noordwijk, The Netherlands, 2012; ISBN 978-92-9221-419-7. [Google Scholar]
- Poilvé, H. Geoland2—BioPar Methods Compendium of MERIS FR Biophysical Products. 2010. Available online: https://www.researchgate.net/publication/265728093_geoland2_-_BioPar_Methods_Compendium_of_MERIS_FR_Biophysical_Products (accessed on 27 May 2021). [CrossRef]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef] [Green Version]
- MacArthur, R.H. Patterns of Species Diversity. Biol. Rev. 1965, 40, 510–533. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017.
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- McCarthy, B.C.; Magurran, A.E. Measuring Biological Diversity. J. Torrey Bot. Soc. 2004, 131, 277. [Google Scholar] [CrossRef] [Green Version]
- Cavender-Bares, J.; Gamon, J.A.; Townsend, P.A. Remote Sensing of Plant Biodiversity; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-030-33157-3. [Google Scholar]
- Feret, J.; Boissieu, F. BiodivMapR: An r Package for A- and Β-diversity Mapping Using Remotely Sensed Images. Methods Ecol. Evol. 2020, 11, 64–70. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gamon, J.A. Remote Sensing of Plant Functional Types: Tansley Review. New Phytol. 2010, 186, 795–816. [Google Scholar] [CrossRef] [PubMed]
- Clerc, S.; Team, M. Sentinel-2 Data Quality Report (S2-PDGS-MPC-DQR). ESA Technical Report. 2016. Available online: https://sentinel.esa.int/documents/247904/685211/Sentinel-2+Data+Quality+Report+(DQR)/f42497d3-611f-4165-bcc1-2f81421c646a (accessed on 27 May 2021).
- Rohlf, F.J. An Empirical Comparison of Three Ordination Techniques in Numerical Taxonomy. Syst. Biol. 1972, 21, 271–280. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Koenker, R.; Leorato, S.; Peracchi, F. Distributional vs. Quantile Regression. SSRN Electron. J. 2013, 300, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Cade, B.S.; Noon, B.R. A Gentle Introduction to Quantile Regression for Ecologists. Front. Ecol. Environ. 2003, 1, 412–420. [Google Scholar] [CrossRef]
- John, O. Robustness of Quantile Regression to Outliers. Am. J. Appl. Math. Stat. 2015, 3, 86–88. [Google Scholar] [CrossRef]
- Galili, T. Dendextend: An R Package for Visualizing, Adjusting and Comparing Trees of Hierarchical Clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef] [Green Version]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions. Available online: https://CRAN.R-project.org/package=cluster (accessed on 1 March 2021).
- Helmer, E.H.; Ruzycki, T.S.; Benner, J.; Voggesser, S.M.; Scobie, B.P.; Park, C.; Fanning, D.W.; Ramnarine, S. Detailed Maps of Tropical Forest Types Are within Reach: Forest Tree Communities for Trinidad and Tobago Mapped with Multiseason Landsat and Multiseason Fine-Resolution Imagery. For. Ecol. Manag. 2012, 279, 147–166. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Meave, J.A.; Poorter, L.; Pérez-García, E.A.; Bongers, F. Pathways, Mechanisms and Predictability of Vegetation Change during Tropical Dry Forest Succession. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 267–275. [Google Scholar] [CrossRef]
- Schoonmaker, P.; McKee, A. Species Composition and Diversity During Secondary Succession of Coniferous Forests in the Western Cascade Mountains of Oregon. For. Sci. 1988, 34, 960–979. [Google Scholar] [CrossRef]
- Toky, O.P.; Ramakrishnan, P.S. Secondary Succession Following Slash and Burn Agriculture in North-Eastern India: I. Biomass, Litterfall and Productivity. J. Ecol. 1983, 71, 735–745. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Gamon, J.A.; Cavender-Bares, J.; Townsend, P.A.; Zygielbaum, A.I. The Spatial Sensitivity of the Spectral Diversity-Biodiversity Relationship: An Experimental Test in a Prairie Grassland. Ecol. Appl. 2018, 28, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Aguirre-Gutiérrez, J.; Rifai, S.; Shenkin, A.; Oliveras, I.; Bentley, L.P.; Svátek, M.; Girardin, C.A.J.; Both, S.; Riutta, T.; Berenguer, E.; et al. Pantropical Modelling of Canopy Functional Traits Using Sentinel-2 Remote Sensing Data. Remote Sens. Environ. 2021, 252, 112122. [Google Scholar] [CrossRef]
- Ma, X.; Mahecha, M.D.; Migliavacca, M.; van der Plas, F.; Benavides, R.; Ratcliffe, S.; Kattge, J.; Richter, R.; Musavi, T.; Baeten, L.; et al. Inferring Plant Functional Diversity from Space: The Potential of Sentinel-2. Remote Sens. Environ. 2019, 233, 111368. [Google Scholar] [CrossRef]
- Sandor, M.E.; Chazdon, R.L. Remnant Trees Affect Species Composition but Not Structure of Tropical Second-Growth Forest. PLoS ONE 2014, 9, e83284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallis, C.I.B.; Brehm, G.; Donoso, D.A.; Fiedler, K.; Homeier, J.; Paulsch, D.; Süßenbach, D.; Tiede, Y.; Brandl, R.; Farwig, N.; et al. Remote Sensing Improves Prediction of Tropical Montane Species Diversity but Performance Differs among Taxa. Ecol. Indic. 2017, 83, 538–549. [Google Scholar] [CrossRef]
- García-Montiel, D.C.; Scatena, F.N. The Effect of Human Activity on the Structure and Composition of a Tropical Forest in Puerto Rico. For. Ecol. Manag. 1994, 63, 57–78. [Google Scholar] [CrossRef]
- Riswan, S.; Kenworthy, J.B.; Kartawinata, K. The Estimation of Temporal Processes in Tropical Rain Forest: A Study of Primary Mixed Dipterocarp Forest in Indonesia. J. Trop. Ecol. 1985, 1, 171–182. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Chazdon, R.L.; Denslow, J.S.; Dupuy, J.M.; Anderson, L. Structure and Floristics of Secondary and Old-Growth Forest Stands in Lowland Costa Rica. Plant Ecol. 1997, 132, 107–120. [Google Scholar] [CrossRef]
- Norden, N.; Chazdon, R.L.; Chao, A.; Jiang, Y.-H.; Vílchez-Alvarado, B. Resilience of Tropical Rain Forests: Tree Community Reassembly in Secondary Forests. Ecol. Lett. 2009, 12, 385–394. [Google Scholar] [CrossRef]
- Ganivet, E.; Bloomberg, M. Towards Rapid Assessments of Tree Species Diversity and Structure in Fragmented Tropical Forests: A Review of Perspectives Offered by Remotely-Sensed and Field-Based Data. For. Ecol. Manag. 2019, 432, 40–53. [Google Scholar] [CrossRef]
- Araújo-Santos, I.; Morante-Filho, J.C.; Oliveira, S.; Cabral, J.P.; Rocha-Santos, L.; Cassano, C.R.; Faria, D.; Benchimol, M. Seed Rain in Cocoa Agroforests Is Induced by Effects of Forest Loss on Frugivorous Birds and Management Intensity. Agric. Ecosyst. Environ. 2021, 313, 107380. [Google Scholar] [CrossRef]
- Cabral, J.P.; Faria, D.; Morante-Filho, J.C. Landscape Composition Is More Important than Local Vegetation Structure for Understory Birds in Cocoa Agroforestry Systems. For. Ecol. Manag. 2021, 481, 118704. [Google Scholar] [CrossRef]
- Cubiña, A.; Aide, T.M. The Effect of Distance from Forest Edge on Seed Rain and Soil Seed Bank in a Tropical Pasture. Biotropica 2001, 33, 260–267. [Google Scholar] [CrossRef]
- Hooper, E.R.; Legendre, P.; Condit, R. Factors Affecting Community Composition of Forest Regeneration in Deforested, Abandoned Land in Panama. Ecology 2004, 85, 3313–3326. [Google Scholar] [CrossRef] [Green Version]
- Hyatt, L.A.; Casper, B.B. Seed Bank Formation during Early Secondary Succession in a Temperate Deciduous Forest. J. Ecol. 2000, 88, 516–527. [Google Scholar] [CrossRef]
- Rolim, S.G.; Machado, R.E.; Pillar, V.D. Divergence in a Neotropical Forest during 33 Years of Succession Following Clear-Cutting. J. Veg. Sci. 2017, 28, 495–503. [Google Scholar] [CrossRef]
- Franklin, J.F.; Spies, T.A.; Pelt, R.V.; Carey, A.B.; Thornburgh, D.A.; Berg, D.R.; Lindenmayer, D.B.; Harmon, M.E.; Keeton, W.S.; Shaw, D.C.; et al. Disturbances and Structural Development of Natural Forest Ecosystems with Silvicultural Implications, Using Douglas-Fir Forests as an Example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]









| Site | Age (Years) | Altitude (m) | Site | Age (Years) | Altitude (m) |
|---|---|---|---|---|---|
| AME | 0 | 207 | VCR | 65 | 352 |
| UpLop1 | 0 | 550 | BR1 | 70 | 321 |
| OA | 0 | 100 | UC1 | 75 | 158 |
| SASC | 0 | 92 | OT1 | 80 | 185 |
| ERE | 0 | 6 | MW8 | 80 | 244 |
| Lop1 | 0 | 167 | LAL | 80 | 471 |
| BSC | 0 | 52 | MSB | 100 | 267 |
| Sim1 | 25 | 211 | LHC | 100 | 360 |
| MSBT | 25 | 467 | SCA | 100 | 217 |
| BST | 30 | 100 | GL | 100 | 373 |
| CMA | 40 | 122 | LLP | 200 | 528 |
| CH | 40 | 90 | LALP | 200 | 550 |
| BR2 | 45 | 334 | NRGS | 200 | 297 |
| OT2 | 50 | 221 | VCRP | 200 | 148 |
| C1 | 50 | 149 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chraibi, E.; Arnold, H.; Luque, S.; Deacon, A.; Magurran, A.E.; Féret, J.-B. A Remote Sensing Approach to Understanding Patterns of Secondary Succession in Tropical Forest. Remote Sens. 2021, 13, 2148. https://doi.org/10.3390/rs13112148
Chraibi E, Arnold H, Luque S, Deacon A, Magurran AE, Féret J-B. A Remote Sensing Approach to Understanding Patterns of Secondary Succession in Tropical Forest. Remote Sensing. 2021; 13(11):2148. https://doi.org/10.3390/rs13112148
Chicago/Turabian StyleChraibi, Eric, Haley Arnold, Sandra Luque, Amy Deacon, Anne E. Magurran, and Jean-Baptiste Féret. 2021. "A Remote Sensing Approach to Understanding Patterns of Secondary Succession in Tropical Forest" Remote Sensing 13, no. 11: 2148. https://doi.org/10.3390/rs13112148
APA StyleChraibi, E., Arnold, H., Luque, S., Deacon, A., Magurran, A. E., & Féret, J. -B. (2021). A Remote Sensing Approach to Understanding Patterns of Secondary Succession in Tropical Forest. Remote Sensing, 13(11), 2148. https://doi.org/10.3390/rs13112148