Assessing Leaf Biomass of Agave sisalana Using Sentinel-2 Vegetation Indices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Plots and Leaf Biomass Estimation
2.3. Sentinel-2 Satellite Image
2.4. Vegetation Index Calculation
2.5. Modelling Biomass with Vegetation Indices
3. Results
3.1. Relationship between Biomass and Vegetation Indices
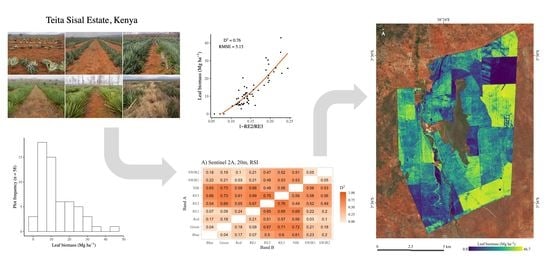
3.2. Biomass Map
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sataya, B.; Maiti, R. Bast and Leaf Fibre Crops: Kenaf, Hemp, Jute, Agave, etc. In Biofuel Crops: Production, Physiology and Genetics; Singh, B.P., Ed.; CABI: Oxfordshire, UK, 2013; pp. 292–308. [Google Scholar] [CrossRef]
- Davis, S.C.; Stephen, P.L. Sisal/Agave. In Industrial Crops: Breeding for Bioenergy and Bioproducts; Von Cruz, M.V., Dierig, D.A., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2015; pp. 339–345. [Google Scholar] [CrossRef]
- Blunden, G.; Yi, Y.; Jewers, K. The comparative leaf anatomy of Agave, Beschorneria, Doryanthes and Furcraea species (Agavaceae: Agaveae). Bot. J. Linn. Soc. 1973, 66, 157–179. [Google Scholar] [CrossRef]
- Stewart, J.R. Agave as a model CAM crop system for a warming and drying world. Front. Plant Sci. 2015, 6, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, P.; Gupta, M.K. Sisal (Agave sisalana) fibre and its polymer-based composites: A review on current developments. J. Reinf. Plast. Compos. 2017, 36, 1759–1780. [Google Scholar] [CrossRef]
- Santos, J.D.G.; Vieira, I.J.C.; Braz-Filho, R.; Branco, A. Chemicals from Agave sisalana Biomass: Isolation and Identification. Int. J. Mol. Sci. 2015, 16, 8761–8771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organisation of the United Nations, FAOSTATS. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 22 September 2020).
- Davis, S.C.; Dohleman, F.G.; Long, S.P. The global potential for Agave as a biofuel feedstock. Glob. Change Biol. Bioenergy 2011, 3, 68–78. [Google Scholar] [CrossRef]
- Niechayev, N.A.; Jones, A.M.; Rosenthal, D.M.; Davis, S.C. A model of environmental limitations on production of Agave americana L. grown as a biofuel crop in semi-arid regions. J. Exp. Bot. 2019, 70, 6549–6559. [Google Scholar] [CrossRef]
- Pérez-Pimienta, J.A.; López-Ortega, M.G.; Sanchez, A. Recent developments in Agave performance as a drought-tolerant biofuel feedstock: Agronomics, characterization, and biorefining. Biofuels Bioprod. Biorefining 2017, 11, 732–748. [Google Scholar] [CrossRef]
- Terrapon-Pfaff, J.C.; Fischedick, M.; Monheim, H. Energy potentials and sustainability—The case of sisal residues in Tanzania. Energy Sustain. Dev. 2012, 16, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Nobel, P.S.; García-moya, E.; Quero, E. High annual productivity of certain agaves and cacti under cultivation. Plant Cell Environ. 1992, 329–335. [Google Scholar] [CrossRef]
- Weiss, M.; Jacob, F.; Duveiller, G. Remote sensing for agricultural applications: A meta-review. Remote Sens. Environ. 2020, 236, 111402. [Google Scholar] [CrossRef]
- Battude, M.; Al Bitar, A.; Morin, D.; Cros, J.; Huc, M.; Sicre, C.M.; Le Dantec, V.; Demarez, V. Estimating maize biomass and yield over large areas using high spatial and temporal resolution Sentinel-2 like remote sensing data. Remote Sens. Environ. 2016, 184, 668–681. [Google Scholar] [CrossRef]
- Gao, F.; Anderson, M.C.; Zhang, X.; Yang, Z.; Alfieri, J.G.; Kustas, W.P.; Mueller, R.; Johnson, D.M.; Prueger, J.H. Toward mapping crop progress at field scales through fusion of Landsat and MODIS imagery. Remote Sens. Environ. 2017, 188, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Kanke, Y.; Tubaña, B.; Dalen, M.; Harrell, D. Evaluation of red and red-edge reflectance-based vegetation indices for rice biomass and grain yield prediction models in paddy fields. Precis. Agric. 2016, 17, 507–530. [Google Scholar] [CrossRef]
- Serrano, L.; Filella, L.; Penuelas, J. Remote Sensing of Biomass and Yield of Winter Wheat. Crop. Sci. 2000, 40, 723–731. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhou, X.; Zhu, X.; Dong, Z.; Guo, W. Estimation of biomass in wheat using random forest regression algorithm and remote sensing data. Crop. J. 2016, 4, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Lemus, R.; Lal, R. Bioenergy crops and carbon sequestration. Crit. Rev. Plant Sci. 2005, 24, 1–21. [Google Scholar] [CrossRef]
- Mathew, I.; Shimelis, H.; Mutema, M.; Chaplot, V. What crop type for atmospheric carbon sequestration: Results from a global data analysis. Agric. Ecosyst. Environ. 2017, 243, 34–46. [Google Scholar] [CrossRef]
- Castillo, J.A.A.; Apan, A.A.; Maraseni, T.N.; Salmo, S.G. Estimation and mapping of above-ground biomass of mangrove forests and their replacement land uses in the Philippines using Sentinel imagery. ISPRS J. Photogramm. Remote Sens. 2017, 134, 70–85. [Google Scholar] [CrossRef]
- Sibanda, M.; Mutanga, O.; Rouget, M. Examining the potential of Sentinel-2 MSI spectral resolution in quantifying above ground biomass across different fertilizer treatments. ISPRS J. Photogramm. Remote Sens. 2015, 110, 55–65. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Kooistra, L.; van den Brande, M.M.M. Using Sentinel-2 data for retrieving LAI and leaf and canopy chlorophyll content of a potato crop. Remote Sens. 2017, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus hippocastanum L. and Acer platanoides L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant. Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Frampton, W.J.; Dash, J.; Watmough, G.; Milton, E.J. Evaluating the capabilities of Sentinel-2 for quantitative estimation of biophysical variables in vegetation. ISPRS J. Photogramm. Remote Sens. 2013, 82, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Mutanga, O.; Skidmore, A.K. Narrow band vegetation indices overcome the saturation problem in biomass estimation. Int. J. Remote Sens. 2004, 25, 3999–4014. [Google Scholar] [CrossRef]
- Revill, A.; Florence, A.; MacArthur, A.; Hoad, S.P.; Rees, R.M.; Williams, M. The value of Sentinel-2 spectral bands for the assessment of winter wheat growth and development. Remote Sens. 2019, 11, 2050. [Google Scholar] [CrossRef] [Green Version]
- Kross, A.; McNairn, H.; Lapen, D.; Sunohara, M.; Champagne, C. Assessment of RapidEye vegetation indices for estimation of leaf area index and biomass in corn and soybean crops. Int. J. Appl. Earth Obs. Geoinf. 2015, 34. [Google Scholar] [CrossRef] [Green Version]
- Marshall, M.; Thenkabail, P. Advantage of hyperspectral EO-1 Hyperion over multispectral IKONOS, GeoEye-1, WorldView-2, Landsat ETM+, and MODIS vegetation indices in crop biomass estimation. ISPRS J. Photogramm. Remote Sens. 2015, 108, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Prabhakara, K.; Hively, W.D.; McCarty, G.W. Evaluating the relationship between biomass, percent groundcover and remote sensing indices across six winter cover crop fields in Maryland, United States. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Pellikka, P.K.E.; Clark, B.J.F.; Gosa, A.G.; Himberg, N.; Hurskainen, P.; Maeda, E.; Mwang’ombe, J.; Omoro, L.M.A.; Siljander, M. Agricultural Expansion and Its Consequences in the Taita Hills, Kenya. Dev. Earth Surf. Process. 2013, 16, 165–179. [Google Scholar] [CrossRef] [Green Version]
- Platts, P.J.; Burgess, N.D.; Gereau, R.E.; Lovett, J.C.; Marshall, A.R.; McClean, C.J.; Pellikka, P.K.E.; Swetnam, R.D.; Marchant, R. Delimiting tropical mountain ecoregions for conservation. Environ. Conserv. 2011, 38, 312–324. [Google Scholar] [CrossRef] [Green Version]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Pellikka, P.K.E.; Heikinheimo, V.; Hietanen, J.; Schäfer, E.; Siljander, M.; Heiskanen, J. Impact of land cover change on aboveground carbon stocks in Afromontane landscape in Kenya. Appl. Geogr. 2018, 94, 178–189. [Google Scholar] [CrossRef]
- Wachiye, S.; Merbold, L.; Vesala, T.; Rinne, J.; Räsänen, M.; Leitner, S.; Pellikka, P.K.E. Soil Greenhouse Gas Emissions under Different Land-Use Types in Savanna Ecosystems of Kenya. Biogeosciences 2019, 17, 2149–2167. [Google Scholar] [CrossRef] [Green Version]
- Mrombo, E.; Teita Sisal Estate, Taita-Taveta, Kenya. Personal communication, 2019.
- Nobel, P.S. Par, Water, and Temperature Limitations on the Productivity of Cultivated Agave fourcroydes (Henequen). J. Appl. Ecol. 1985, 157–173. [Google Scholar] [CrossRef]
- Vuorinne, I.; Heiskanen, J.; Mwangala, L.; Maghenda, M.; Pellikka, P. Estimating Agave Sisalana Leaf Biomass and Productivity in a Semi-arid Environment. (under review).
- Wachiye, S.; Merbold, L.; Vesala, T.; Rinne, J.; Leitner, S.; Räsänen, M.; Vuorinne, I.; Heiskanen, J.; Pellikka, P. Soil Greenhouse Gas Emissions from a Sisal Chronosequence in Kenya. (under review).
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation. 2019. Available online: http://qgis.org (accessed on 1 November 2019).
- Baskerville, G.L. Use of Logarithmic Regression in the Estimation of Plant Biomass: Reply. Can. J. For. Res. 1974, 4, 149. [Google Scholar] [CrossRef]
- Copernicus Open Access Hub. Available online: https://scihub.copernicus (accessed on 1 November 2019).
- Sen2Cor Configuration and User Manual. Available online: http://step.esa.int/thirdparties/sen2cor/2.8.0/docs/S2-PDGS-MPC-L2A-SUM-V2.8.pdf (accessed on 28 December 2020).
- Sentinel-2 User Handbook. Available online: https://sentinels.copernicus.eu/documents/247904/685211/Sentinel-2_User_Handbook (accessed on 1 April 2020).
- Python Software Foundation. Python Language Reference, Version 3.6. Available online: http://www.python.org (accessed on 1 April 2020).
- Horler, D.N.H.; Dockray, M.; Barber, J. The red edge of plant leaf reflectance. Int. J. Remote Sens. 1983, 4, 273–288. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Huete, A.; Didan, J.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.R.E.; Thompson, T.; Lascano, R.J.; et al. Coincident detection of crop water stress, nitrogen status and canopy density using ground based multispectral data. In Proceedings of the 5th International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Rouse, W.; Haas, R.H.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. Available online: https://ntrs.nasa.gov/citations/19740022614 (accessed on 1 April 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.4.0; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Taylor & Francis: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Korhonen, L.; Hadi; Packalen, P.; Rautiainen, M. Comparison of Sentinel-2 and Landsat 8 in the estimation of boreal forest canopy cover and leaf area index. Remote Sens. Environ. 2017, 195, 259–274. [Google Scholar] [CrossRef]
- Riihimäki, H.; Luoto, M.; Heiskanen, J. Estimating fractional cover of tundra vegetation at multiple scales using unmanned aerial systems and optical satellite data. Remote Sens. Environ. 2019, 224, 119–132. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Poblete-Olivares, J.; Rivero, L.; Lopatin, J.; Ceballos-Comisso, A.; Galleguillos, M. Using Sentinel-2 and canopy height models to derive a landscape-level biomass map covering multiple vegetation types. Int. J. Appl. Earth Obs. Geoinf. 2021, 94, 102236. [Google Scholar] [CrossRef]
- Ruppert, D. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. J. Am. Stat. Assoc. 2004, 99, 567. [Google Scholar] [CrossRef]
- Ploton, P.; Mortier, F.; Réjou-Méchain, M.; Barbier, N.; Picard, N.; Rossi, V.; Dormann, C.; Cornu, G.; Viennois, G.; Bayol, N.; et al. Spatial validation reveals poor predictive performance of large-scale ecological mapping models. Nat. Commun. 2020, 11, 4540. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Viña, A.; Arkebauer, T.J.; Runquist, D.C.; Keydan, G.; Leavitt, B. Remote Estimation of green leaf biomass in maize canopies. Geophys. Res. Lett. 2003, 30, 1248. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Silva, L. Light Ray Tracing Through a Leaf Cross Section. Appl. Opt. 1973, 12, 2950–2954. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Prudnikova, E.; Savin, I.; Vindeker, G.; Grubina, P.; Shishkonakova, E.; Sharychev, D. Influence of soil background on spectral reflectance of winter wheat crop canopy. Remote Sens. 2019, 11, 1932. [Google Scholar] [CrossRef] [Green Version]
- Viña, A.; Gitelson, A.A.; Nguy-Robertson, A.L.; Peng, Y. Comparison of different vegetation indices for the remote assessment of green leaf area index of crops. Remote Sens. Environ. 2011, 115, 3468–3478. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Skidmore, A.; Atzberger, C.; van Wieren, S. Estimation of vegetation LAI from hyperspectral reflectance data: Effects of soil type and plant architecture. Int. J. Appl. Earth Obs. Geoinf. 2008, 10, 358–373. [Google Scholar] [CrossRef]
- Forkuor, G.; Zoungrana, J.B.B.; Dimobe, K.; Ouattara, B.; Vadrevu, K.P.; Tondoh, J.E. Above-ground biomass mapping in West African dryland forest using Sentinel-1 and 2 datasets—A case study. Remote Sens. Environ. 2020, 235, 111496. [Google Scholar] [CrossRef]
- Liu, J.; Heiskanen, J.; Aynekulu, E.; Maeda, E.E.; Pellikka, P.K.E. Land cover characterization in West Sudanian savannas using seasonal features from annual landsat time series. Remote Sens. 2016, 8, 365. [Google Scholar] [CrossRef] [Green Version]
- Foody, G.M.; Boyd, D.S.; Cutler, M.E.J. Predictive relations of tropical forest biomass from Landsat TM data and their transferability between regions. Remote Sens. Environ. 2003, 85, 463–474. [Google Scholar] [CrossRef]
- Asner, G.P. Cloud cover in Landsat observations of the Brazilian Amazon. Int. J. Remote Sens. 2001, 22, 3855–3862. [Google Scholar] [CrossRef]
- Han, L.; Yang, G.; Dai, H.; Xu, B.; Yang, H.; Feng, H.; Li, Z.; Yang, X. Modeling maize above-ground biomass based on machine learning approaches using UAV remote-sensing data. Plant Methods 2019, 15. [Google Scholar] [CrossRef] [Green Version]
- Piiroinen, R.; Heiskanen, J.; Mõttus, M.; Pellikka, P.K.E. Classification of crops across heterogeneous agricultural landscape in Kenya using AisaEAGLE imaging spectroscopy data. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 1–8. [Google Scholar] [CrossRef]
- Calvario, G.; Sierra, B.; Alarcón, T.E.; Hernandez, C.; Dalmau, O. A multi-disciplinary approach to remote sensing through low-cost UAVs. Sensors 2017, 17, 1411. [Google Scholar] [CrossRef] [Green Version]
- Landmann, T.; Piiroinen, R.; Makori, D.M.; Abdel-Rahman, E.M.; Makau, S.; Pellikka, P.K.E.; Raina, S.K. Application of hyperspectral remote sensing for flower mapping in African savannas. Remote Sens. Environ. 2015, 166, 50–60. [Google Scholar] [CrossRef]










| Min. | 1st. Quantile | Median | Mean | 3rd. Quantile | Max. | |
|---|---|---|---|---|---|---|
| Leaf Biomass (Mg ha−1) | 0.0 | 5.1 | 10.0 | 12.1 | 16.0 | 42.3 |
| Block Age (years) | 0 | 3 | 7 | 7.5 | 13 | 17 |
| Band | Spectral Band | Central Wavelength (nm) | Band Width (nm) | Spatial Resolution |
|---|---|---|---|---|
| B1 | Coastal aerosol | 443 | 20 | 60 |
| B2 | Blue | 490 | 65 | 10 |
| B3 | Green | 560 | 35 | 10 |
| B4 | Red | 665 | 30 | 10 |
| B5 | Red-edge, RE1 | 705 | 15 | 20 |
| B6 | Red-edge, RE2 | 740 | 15 | 20 |
| B7 | Red-edge, RE3 | 783 | 20 | 20 |
| B8 | Near infrared | 842 | 115 | 10 |
| B8A | Near infrared narrow, NIR | 865 | 20 | 20 |
| B9 | Water vapour | 945 | 20 | 60 |
| B10 | Shortwave infrared | 1380 | 30 | 60 |
| B11 | Shortwave infrared, SWIR1 | 1910 | 90 | 20 |
| B12 | Shortwave infrared, SWIR2 | 2190 | 180 | 20 |
| Index | Formula | Reference |
|---|---|---|
| SAVI | ((NIR − Red))/((NIR + Red + 0.16)) | [48] |
| EVI | 2.5 × (NIR − Red)/((NIR + 6Red − 7.5Blue) + 1) | [49] |
| CCCI | ((NIR − RE1)/(NIR + RE1))/((NIR − Red)/(NIR+Red)) | [50] |
| IRECI | (NIR − Red)/(RE1 − RE2) | [51] |
| MCARI | ((RE1-Red) − 0.2(RE1 − Green))(RE1/Red) | [26] |
| NDVI | (NIR − Red)/(NIR + Red) | [52] |
| Index | D2 | MAE | MAE | RMSE | RMSE | nRMSE | nRMSE |
|---|---|---|---|---|---|---|---|
| LOOCV | LBOCV | LOOCV | LBOCV | LOOCV | LBOCV | ||
| (Mg ha−1) | (Mg ha−1) | (Mg ha−1) | (Mg ha−1) | (%) | (%) | ||
| RE2/RE3 | 0.76 | 3.60 | 3.77 | 4.90 | 5.15 | 46 | 49 |
| (RE3 − RE2)/(RE3 + RE2) | 0.76 | 3.66 | 3.80 | 4.97 | 5.17 | 47 | 49 |
| NIR/Green | 0.72 | 3.89 | 4.18 | 5.11 | 5.42 | 48 | 51 |
| (NIR − Green)/(NIR + Green) | 0.72 | 3.90 | 4.19 | 5.12 | 5.41 | 48 | 51 |
| 1/RE3–1/RE1 | 0.68 | 4.11 | 4.49 | 5.80 | 6.25 | 54 | 58 |
| 1/RE3–1/Green | 0.67 | 4.17 | 4.45 | 5.66 | 6.11 | 53 | 57 |
| MCARI | 0.64 | 4.34 | 4.54 | 5.93 | 6.20 | 56 | 58 |
| IRECI | 0.64 | 4.37 | 4.61 | 5.97 | 6.24 | 56 | 59 |
| EVI | 0.62 | 4.37 | 4.58 | 5.92 | 6.26 | 56 | 59 |
| SAVI | 0.61 | 4.37 | 4.62 | 6.02 | 6.33 | 57 | 60 |
| CCCI | 0.60 | 4.26 | 4.38 | 6.09 | 6.31 | 57 | 60 |
| NDVI | 0.58 | 4.43 | 4.65 | 6.26 | 6.57 | 59 | 61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuorinne, I.; Heiskanen, J.; Pellikka, P.K.E. Assessing Leaf Biomass of Agave sisalana Using Sentinel-2 Vegetation Indices. Remote Sens. 2021, 13, 233. https://doi.org/10.3390/rs13020233
Vuorinne I, Heiskanen J, Pellikka PKE. Assessing Leaf Biomass of Agave sisalana Using Sentinel-2 Vegetation Indices. Remote Sensing. 2021; 13(2):233. https://doi.org/10.3390/rs13020233
Chicago/Turabian StyleVuorinne, Ilja, Janne Heiskanen, and Petri K. E. Pellikka. 2021. "Assessing Leaf Biomass of Agave sisalana Using Sentinel-2 Vegetation Indices" Remote Sensing 13, no. 2: 233. https://doi.org/10.3390/rs13020233
APA StyleVuorinne, I., Heiskanen, J., & Pellikka, P. K. E. (2021). Assessing Leaf Biomass of Agave sisalana Using Sentinel-2 Vegetation Indices. Remote Sensing, 13(2), 233. https://doi.org/10.3390/rs13020233