Satellite-Based Observations Reveal the Altitude-Dependent Patterns of SIFyield and Its Sensitivity to Ambient Temperature in Tibetan Meadows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Satellite-Based SIF Dataset
2.3. Datasets of Environment Variables
2.4. DEM Data
2.5. Other Auxiliary Datasets
2.6. Statistical Analysis
3. Results
3.1. Altitudinal Patterns of SIFyield Changes
3.1.1. Altitudinal Variation in SIFyield
3.1.2. Altitude-Dependent SIFyield Changes at Interannual Scale
3.2. Altitudinal Patterns of Changes in SIFyield Temperature Sensitivity
3.2.1. Seasonal and Interannual Temperature Sensitivity of SIFyield
3.2.2. Spatial Distribution of Temperature Sensitivity of SIFyield
4. Discussion
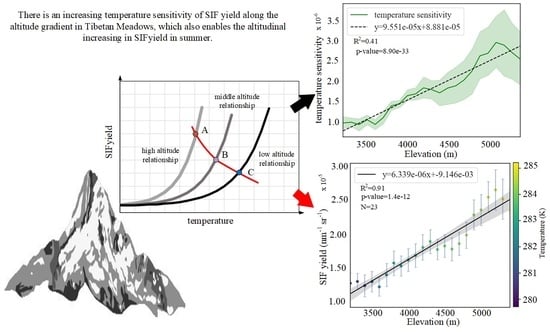
4.1. Possible Altitudinal Increase in SIFyield Observed in Summer
4.2. Interpretation of Patterns Observed at Different Scales
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Körner, C. The use of “altitude” in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Prentice, I.C.; Davis, T.W.; Keenan, T.F.; Wright, I.J.; Peng, C. Photosynthetic responses to altitude: An explanation based on optimality principles. New Phytol. 2017, 213, 976–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.W.; He, X.F.; Chen, J.G.; Sun, H.; Körner, C.; Yang, Y. Elevation-specific responses of phenology in evergreen oaks from their low-dry to their extreme high-cold range limits in the SE Himalaya. Alp. Bot. 2021. [Google Scholar] [CrossRef]
- Gale, J. Availability of carbon dioxide for photosynthesis at high altitudes: Theoretical considerations. Ecology 1972, 53, 494–497. [Google Scholar] [CrossRef]
- Friend, A.D.; Woodward, F.I.; Switsur, V.R. Field measurements of photosynthesis, stomatal conductance, leaf nitrogen and δ 13 C along altitudinal gradients in Scotland. Funct. Ecol. 1989, 117–122. [Google Scholar] [CrossRef]
- Friend, A.D.; Woodward, F.I. Evolutionary and ecophysiological responses of mountain plants to the growing season environment. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 1990; Volume 20, pp. 59–124. [Google Scholar]
- Machino, S.; Nagano, S.; Hikosaka, K. The latitudinal and altitudinal variations in the biochemical mechanisms of temperature dependence of photosynthesis within Fallopia japonica. Environ. Exp. Bot. 2021, 181, 104248. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Liang, L.; Donnelly, A.; Park, I.; Schwartz, M.D. Effects of elevation on spring phenological sensitivity to temperature in Tibetan Plateau grasslands. Chin. Sci. Bull. 2014, 59, 4856–4863. [Google Scholar] [CrossRef]
- Luo, T.; Luo, J.; Pan, Y. Leaf traits and associated ecosystem characteristics across subtropical and timberline forests in the Gongga Mountains, Eastern Tibetan Plateau. Oecologia 2005, 142, 261–273. [Google Scholar] [CrossRef]
- Buchner, O.; Neuner, G. Variability of heat tolerance in alpine plant species measured at different altitudes. Arctic Antarct. Alp. Res. 2003, 35, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Shi, P.; Li, W.; He, Y.; Zhang, X.; Shen, Z.; Chai, S. Changes in individual plant traits and biomass allocation in alpine meadow with elevation variation on the Qinghai-Tibetan Plateau. Sci. China Life Sci. 2010. [Google Scholar] [CrossRef]
- Luo, T.; Brown, S.; Pan, Y.; Shi, P.; Ouyang, H.; Yu, Z.; Zhu, H. Root biomass along subtropical to alpine gradients: Global implication from Tibetan transect studies. For. Ecol. Manag. 2005, 206, 349–363. [Google Scholar] [CrossRef]
- Pellissier, L.; Fournier, B.; Guisan, A.; Vittoz, P. Plant traits co-vary with altitude in grasslands and forests in the European Alps. Plant Ecol. 2010. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Bloomfield, K.J.; Prentice, I.C. A theory of plant function helps to explain leaf-trait and productivity responses to elevation. New Phytol. 2020. [Google Scholar] [CrossRef]
- Wu, J.M.; Shi, Z.; Liu, S.; Centritto, M.; Cao, X.; Zhang, M.; Zhao, G. Photosynthetic capacity of male and female Hippophae rhamnoides plants along an elevation gradient in eastern Qinghai-Tibetan Plateau, China. Tree Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- García-Plazaola, J.I.; Rojas, R.; Christie, D.A.; Coopman, R.E. Photosynthetic responses of trees in high-elevation forests: Comparing evergreen species along an elevation gradient in the Central Andes. AoB Plants 2015, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streb, P.; Shang, W.; Feierabend, J.; Bligny, R. Divergent strategies of photoprotection in high-mountain plants. Planta 1998, 207, 313–324. [Google Scholar] [CrossRef]
- Schimel, D.; Pavlick, R.; Fisher, J.B.; Asner, G.P.; Saatchi, S.; Townsend, P.; Miller, C.; Frankenberg, C.; Hibbard, K.; Cox, P. Observing terrestrial ecosystems and the carbon cycle from space. Glob. Chang. Biol. 2015, 21, 1762–1776. [Google Scholar] [CrossRef]
- Frankenberg, C.; Fisher, J.B.; Worden, J.; Badgley, G.; Saatchi, S.S.; Lee, J.E.; Toon, G.C.; Butz, A.; Jung, M.; Kuze, A.; et al. New global observations of the terrestrial carbon cycle from GOSAT: Patterns of plant fluorescence with gross primary productivity. Geophys. Res. Lett. 2011. [Google Scholar] [CrossRef] [Green Version]
- Joiner, J.; Guanter, L.; Lindstrot, R.; Voigt, M.; Vasilkov, A.P.; Middleton, E.M.; Huemmrich, K.F.; Yoshida, Y.; Frankenberg, C. Global monitoring of terrestrial chlorophyll fluorescence from moderate-spectral-resolution near-infrared satellite measurements: Methodology, simulations, and application to GOME-2. Atmos. Meas. Tech. 2013, 6, 2803–2823. [Google Scholar] [CrossRef] [Green Version]
- Guanter, L.; Frankenberg, C.; Dudhia, A.; Lewis, P.E.; Gómez-Dans, J.; Kuze, A.; Suto, H.; Grainger, R.G. Retrieval and global assessment of terrestrial chlorophyll fluorescence from GOSAT space measurements. Remote Sens Environ. 2012. [Google Scholar] [CrossRef]
- Joiner, J.; Yoshida, Y.; Vasilkov, A.P.; Yoshida, Y.; Corp, L.A.; Middleton, E.M. First observations of global and seasonal terrestrial chlorophyll fluorescence from space. Biogeosciences 2011. [Google Scholar] [CrossRef] [Green Version]
- Köhler, P.; Guanter, L.; Joiner, J. A linear method for the retrieval of sun-induced chlorophyll fluorescence from GOME-2 and SCIAMACHY data. Atmos. Meas. Tech. 2015, 8, 2589–2608. [Google Scholar] [CrossRef] [Green Version]
- Köhler, P.; Frankenberg, C.; Magney, T.S.; Guanter, L.; Joiner, J.; Landgraf, J. Global retrievals of solar-induced chlorophyll fluorescence with TROPOMI: First results and intersensor comparison to OCO-2. Geophys. Res. Lett. 2018, 45, 10456–10463. [Google Scholar] [CrossRef] [Green Version]
- Guanter, L.; Alonso, L.; Gómez-Chova, L.; Amorós-López, J.; Vila, J.; Moreno, J. Estimation of solar-induced vegetation fluorescence from space measurements. Geophys. Res. Lett. 2007. [Google Scholar] [CrossRef]
- Wang, C.; Guan, K.; Peng, B.; Chen, M.; Jiang, C.; Zeng, Y.; Wu, G.; Wang, S.; Wu, J.; Yang, X.; et al. Satellite footprint data from OCO-2 and TROPOMI reveal significant spatio-temporal and inter-vegetation type variabilities of solar-induced fluorescence yield in the US Midwest. Remote Sens. Environ. 2020, 241, 111728. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, B.; Li, W.; Zhang, Q. Impacts of drought and heatwave on the terrestrial ecosystem in China as revealed by satellite solar-induced chlorophyll fluorescence. Sci. Total Environ. 2019, 693, 133627. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Guanter, L.; Guan, K.; You, L.; Huete, A.; Ju, W.; Zhang, Y. Satellite sun-induced chlorophyll fluorescence detects early response of winter wheat to heat stress in the Indian Indo-Gangetic Plains. Glob. Chang. Biol. 2018, 24, 4023–4037. [Google Scholar] [CrossRef] [Green Version]
- Pepin, N.C.; Lundquist, J.D. Temperature trends at high elevations: Patterns across the globe. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef] [Green Version]
- Rangwala, I.; Miller, J.R. Climate change in mountains: A review of elevation-dependent warming and its possible causes. Clim. Chang. 2012, 114, 527–547. [Google Scholar] [CrossRef]
- Flexas, J.; Loreto, F.; Medrano, H. Terrestrial Photosynthesis in a Changing Environment: A Molecular, Physiological, and Ecological Approach; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Barber, J.; Andersson, B. Too much of a good thing: Light can be bad for photosynthesis. Trends Biochem. Sci. 1992, 17, 61–66. [Google Scholar] [CrossRef]
- Ball, M.C.; Hodges, V.S.; Laughlin, G.P. Cold-induced photoinhibition limits regeneration of snow gum at tree-line. Funct. Ecol. 1991, 663–668. [Google Scholar] [CrossRef]
- Ensminger, I.; Busch, F.; Huner, N.P.A. Photostasis and cold acclimation: Sensing low temperature through photosynthesis. Physiol. Plant. 2006, 126, 28–44. [Google Scholar] [CrossRef]
- Huner, N.P.A.; Öquist, G.; Sarhan, F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998, 3, 224–230. [Google Scholar] [CrossRef]
- Cannone, N.; Sgorbati, S.; Guglielmin, M. Unexpected impacts of climate change on alpine vegetation. Front. Ecol. Environ. 2007, 5, 360–364. [Google Scholar] [CrossRef] [Green Version]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate change impacts in alpine environments. Geogr. Compass 2010, 4, 1133–1153. [Google Scholar] [CrossRef] [Green Version]
- Duan, A.; Xiao, Z. Does the climate warming hiatus exist over the Tibetan Plateau? Sci. Rep. 2015, 5, 13711. [Google Scholar] [CrossRef]
- Liu, X.; Chen, B. Climatic warming in the Tibetan Plateau during recent decades. Int. J. Climatol. J. R. Meteorol. Soc. 2000, 20, 1729–1742. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, D.; Zhang, Z.; Yu, E. The impact of regional uplift of the Tibetan Plateau on the Asian monsoon climate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 417, 137–150. [Google Scholar] [CrossRef]
- Liu, X.; Yin, Z.-Y. Sensitivity of East Asian monsoon climate to the uplift of the Tibetan Plateau. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002, 183, 223–245. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Kutzbach, J.E.; Liu, Z.; An, Z.; Li, L. The Tibetan Plateau as amplifier of orbital-scale variability of the East Asian monsoon. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef] [Green Version]
- Harris, N. The elevation history of the Tibetan Plateau and its implications for the Asian monsoon. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 241, 4–15. [Google Scholar] [CrossRef]
- Kang, S.; Xu, Y.; You, Q.; Flügel, W.-A.; Pepin, N.; Yao, T. Review of climate and cryospheric change in the Tibetan Plateau. Environ. Res. Lett. 2010, 5, 15101. [Google Scholar] [CrossRef]
- Harris, R.B. Rangeland degradation on the Qinghai-Tibetan plateau: A review of the evidence of its magnitude and causes. J. Arid. Environ. 2010, 74, 1–12. [Google Scholar] [CrossRef]
- Fang, X.; Han, Y.; Ma, J.; Song, L.; Yang, S.; Zhang, X. Dust storms and loess accumulation on the Tibetan Plateau: A case study of dust event on 4 March 2003 in Lhasa. Chin. Sci. Bull. 2004, 49, 953–960. [Google Scholar] [CrossRef]
- Duveiller, G.; Filipponi, F.; Walther, S.; Köhler, P.; Frankenberg, C.; Guanter, L.; Cescatti, A. A spatially downscaled sun-induced fluorescence global product for enhanced monitoring of vegetation productivity. Earth Syst. Sci. Data 2020, 12, 1101–1116. [Google Scholar] [CrossRef]
- Joiner, J.; Yoshida, Y.; Guanter, L.; Middleton, E.M. New methods for retrieval of chlorophyll red fluorescence from hyper-spectral satellite instruments: Simulations and application to GOME-2 and SCIAMACHY. Atmos. Meas. Tech. Discuss. 2016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Joiner, J.; Gentine, P.; Zhou, S. Reduced solar-induced chlorophyll fluorescence from GOME-2 during Amazon drought caused by dataset artifacts. Glob. Chang. Biol. 2018, 24, 2229–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liangyun, L.; Yan, M.; Ruonan, C. Global degradation corrected 0.05 degree GOME-2 SIF datasets (derived from PK datasets) [Dataset]. Zenodo 2020. [Google Scholar] [CrossRef]
- He, J.; Yang, K.; Tang, W.; Lu, H.; Qin, J.; Chen, Y.Y.; Li, X. The first high-resolution meteorological forcing dataset for land process studies over China. Sci. Data 2020, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; He, J.; Tang, W.J.; Qin, J.; Cheng, C.C.K. On downward shortwave and longwave radiations over high altitude regions: Observation and modeling in the Tibetan Plateau. Agric. For. Meteorol. 2010, 150, 38–46. [Google Scholar] [CrossRef]
- Jarvis, A.; Reuter, H.I.; Nelson, A.; Guevara, E. Guevara, 2008, Hole-filled seamless SRTM data V4. International Centre for Tropical Agriculture (CIAT). Available online: http://srtm.csi.cgiar.org (accessed on 1 September 2020).
- Reuter, H.I.; Nelson, A.; Jarvis, A. An evaluation of void filling interpolation methods for SRTM data. Int. J. Geogr. Inf. Sci. 2007, 21, 983–1008. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, B.Y.; Zheng, D. Datasets of the Boundary and Area of the Tibetan Plateau [DB/OL]. Glob. Chang. Data Repos. 2014. [Google Scholar] [CrossRef]
- Xiao, Z.; Liang, S.; Sun, R.; Wang, J.; Jiang, B. Estimating the fraction of absorbed photosynthetically active radiation from the MODIS data based GLASS leaf area index product. Remote Sens. Environ. 2015, 171, 105–117. [Google Scholar] [CrossRef]
- Xiao, Z.; Liang, S.; Sun, R. Evaluation of three long time series for global fraction of absorbed photosynthetically active radiation (fapar) products. IEEE Trans. Geosci. Remote Sens. 2018, 56, 5509–5524. [Google Scholar] [CrossRef]
- Editorial Committee of the Chinese Vegetation Map of the Chinese Academy of Sciences. 1980 1:1 million vegetation map of China. Institute of Botany, Chinese Academy of Sciences. Available online: http://hdl.pid21.cn/21.86109/casearth.5c19a5680600cf2a3c557b6b (accessed on 1 September 2020).
- Guanter, L.; Zhang, Y.; Jung, M.; Joiner, J.; Voigt, M.; Berry, J.A.; Frankenberg, C.; Huete, A.R.; Zarco-Tejada, P.; Lee, J.E.; et al. Global and time-resolved monitoring of crop photosynthesis with chlorophyll fluorescence. Proc. Natl. Acad. Sci. USA 2014, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, H.B. Nonparametric Tests Against Trend. Econometrica 1945. [Google Scholar] [CrossRef]
- Bevan, J.M.; Kendall, M.G. Rank Correlation Methods. J. R. Stat. Soc. 1971. [Google Scholar] [CrossRef]
- Magaña Ugarte, R.; Escudero, A.; Gavilán, R.G. Metabolic and physiological responses of Mediterranean high-mountain and alpine plants to combined abiotic stresses. Physiol Plant. 2019, 165, 403–412. [Google Scholar] [CrossRef]
- Terashima, I.; Masuzawa, T.; Ohba, H.; Yokoi, Y. Is photosynthesis suppressed at higher elevations due to low CO2 pressure? Ecology 1995, 76, 2663–2668. [Google Scholar] [CrossRef]
- Bresson, C.C.; Kowalski, A.S.; Kremer, A.; Delzon, S. Evidence of altitudinal increase in photosynthetic capacity: Gas exchange measurements at ambient and constant CO2 partial pressures. Ann. For. Sci. 2009, 66, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Siegwolf, R.T.W.; Durka, W.; Körner, C. Phylogenetically balanced evidence for structural and carbon isotope responses in plants along elevational gradients. Oecologia 2010, 162, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Korner, C.; Diemer, M. In situ photosynthetic responses to light, temperature and carbon dioxide in herbaceous plants from low and high altitude. Funct. Ecol. 1987, 179–194. [Google Scholar] [CrossRef]
- Reed, C.C.; Loik, M.E. Water relations and photosynthesis along an elevation gradient for Artemisia tridentata during an historic drought. Oecologia 2016, 181, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.; Liu, S.; Liu, X.; Centritto, M. Altitudinal variation in photosynthetic capacity, diffusional conductance and δ13C of butterfly bush (Buddleja davidii) plants growing at high elevations. Physiol Plant. 2006, 128, 722–731. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Field, C.B.; Matson, P.A. Variation in foliar δ 13 C in Hawaiian Metrosideros polymorpha: A case of internal resistance? Oecologia 1990, 84, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Decker, J.P. Some effects of temperature and carbon dioxide concentration on photosynthesis of Mimulus. Plant Physiol. 1959, 34, 103. [Google Scholar] [CrossRef] [Green Version]
- Billings, W.D.; Clebsch, E.E.C.; Mooney, H.A. Effect of low concentrations of carbon dioxide on photosynthesis rates of two races of Oxyria. Science 1961, 133, 1834. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; Van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef]
- Nichol, C.J.; Drolet, G.; Porcar-Castell, A.; Wade, T.; Sabater, N.; Middleton, E.M.; MacLellan, C.; Levula, J.; Mammarella, I.; Vesala, T. Diurnal and seasonal solar induced chlorophyll fluorescence and photosynthesis in a boreal scots pine canopy. Remote Sens. 2019, 11, 273. [Google Scholar] [CrossRef] [Green Version]
- Raczka, B.; Porcar-Castell, A.; Magney, T.; Lee, J.E.; Köhler, P.; Frankenberg, C.; Grossmann, K.; Logan, B.A.; Stutz, J.; Blanken, P.D.; et al. Sustained Nonphotochemical Quenching Shapes the Seasonal Pattern of Solar-Induced Fluorescence at a High-Elevation Evergreen Forest. J. Geophys. Res. Biogeosci. 2019, 124, 2005–2020. [Google Scholar] [CrossRef]
- Cui, G.; Ji, G.; Liu, S.; Li, B.; Lian, L.; He, W.; Zhang, P. Physiological adaptations of Elymus dahuricus to high altitude on the Qinghai–Tibetan Plateau. Acta Physiol. Plant. 2019, 41, 1–9. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Liu, L.; Liu, X. Satellite-Based Observations Reveal the Altitude-Dependent Patterns of SIFyield and Its Sensitivity to Ambient Temperature in Tibetan Meadows. Remote Sens. 2021, 13, 1400. https://doi.org/10.3390/rs13071400
Chen R, Liu L, Liu X. Satellite-Based Observations Reveal the Altitude-Dependent Patterns of SIFyield and Its Sensitivity to Ambient Temperature in Tibetan Meadows. Remote Sensing. 2021; 13(7):1400. https://doi.org/10.3390/rs13071400
Chicago/Turabian StyleChen, Ruonan, Liangyun Liu, and Xinjie Liu. 2021. "Satellite-Based Observations Reveal the Altitude-Dependent Patterns of SIFyield and Its Sensitivity to Ambient Temperature in Tibetan Meadows" Remote Sensing 13, no. 7: 1400. https://doi.org/10.3390/rs13071400
APA StyleChen, R., Liu, L., & Liu, X. (2021). Satellite-Based Observations Reveal the Altitude-Dependent Patterns of SIFyield and Its Sensitivity to Ambient Temperature in Tibetan Meadows. Remote Sensing, 13(7), 1400. https://doi.org/10.3390/rs13071400