Seasonal Trends in Movement Patterns of Birds and Insects Aloft Simultaneously Recorded by Radar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Radar Data Collection and Processing
2.2. Separating Directional and Non-Directional Movements
2.3. Seasonal Trends in Directional and Non-Directional Traffic Rates
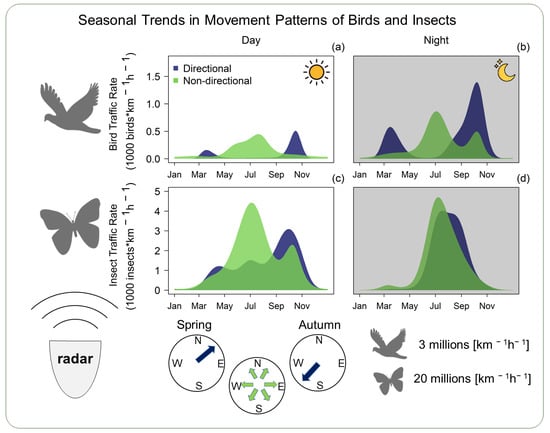
3. Results
3.1. Overview
3.2. Seasonal Trend in Flight Direction and Movement Intensity
4. Discussion
5. Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alerstam, T.; Hedenstrom, A.; Akesson, S. Long-distance migration: Evolution and determinants. Oikos 2003, 103, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Dingle, H.; Drake, V.A. What is migration? Bioscience 2007, 57, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Alexander, R.M. Principles of Animal Locomotion; Princeton University Press: Princeton, NJ, USA, 2003; ISBN 978-0-691-12940-2. [Google Scholar]
- Hahn, S.; Bauer, S.; Liechti, F. The natural link between Europe and Africa—2.1 billion birds on migration. Oikos 2009, 118, 624–626. [Google Scholar] [CrossRef]
- Bauer, S.; Hoye, B.J. Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 2014, 344, 1242552. [Google Scholar] [CrossRef]
- Hu, G.; Lim, K.S.; Horvitz, N.; Clark, S.J.; Reynolds, D.R.; Sapir, N.; Chapman, J.W. Mass seasonal bioflows of high-flying insect migrants. Science 2016, 354, 1584–1587. [Google Scholar] [CrossRef] [Green Version]
- Wotton, K.R.; Gao, B.; Menz, M.H.M.; Morris, R.K.A.; Ball, S.G.; Lim, K.S.; Reynolds, D.R.; Hu, G.; Chapman, J.W. Mass seasonal migrations of hoverflies provide extensive pollination and crop protection services. Curr. Biol. 2019, 29, 2167–2173.e5. [Google Scholar] [CrossRef]
- Diehl, R.H. The airspace is habitat. Trends Ecol. Evol. 2013, 28, 377–379. [Google Scholar] [CrossRef]
- Sanderson, F.J.; Donald, P.F.; Pain, D.J.; Burfield, I.J.; van Bommel, F.P.J. Long-term population declines in Afro-Palearctic migrant birds. Biol. Conserv. 2006, 131, 93–105. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [Green Version]
- Chilson, P.B.; Frick, W.F.; Kelly, J.F.; Liechti, F. Aeroecology: An integrative view of the atmosphere. In Aeroecology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–11. ISBN 9783319685762. [Google Scholar]
- Stefanescu, C.; Páramo, F.; Åkesson, S.; Alarcón, M.; Ávila, A.; Brereton, T.; Carnicer, J.; Cassar, L.F.; Fox, R.; Heliölä, J.; et al. Multi-generational long-distance migration of insects: Studying the painted lady butterfly in the Western Palaearctic. Ecography 2013, 36, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, P.T.; Torres, F.P.; Megías, A.G. Effects of roads on insects: A review. Biodivers. Conserv. 2015, 24, 659–682. [Google Scholar] [CrossRef]
- Grilo, C.; Koroleva, E.; Andrášik, R.; Bíl, M.; González-Suárez, M. Roadkill risk and population vulnerability in European birds and mammals. Front. Ecol. Environ. 2020, 18, 323–328. [Google Scholar] [CrossRef]
- Aschwanden, J.; Stark, H.; Peter, D.; Steuri, T.; Schmid, B.; Liechti, F. Bird collisions at wind turbines in a mountainous area related to bird movement intensities measured by radar. Biol. Conserv. 2018, 220, 228–236. [Google Scholar] [CrossRef]
- Leskinen, M.; Markkula, I.; Koistinen, J.; Pylkkö, P.; Ooperi, S.; Siljamo, P.; Ojanen, H.; Raiskio, S.; Tiilikkala, K. Pest insect immigration warning by an atmospheric dispersion model, weather radars and traps. J. Appl. Entomol. 2011, 135, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Weitnauer, E. Mein Vogel—Aus dem Leben des Mauersegler Apus apus; Basellandschaftlicher Natur- und Vogelschutzverband: Liestal, Switzerland, 1980. [Google Scholar]
- Liechti, F.; Witvliet, W.; Weber, R.; Bächler, E. First evidence of a 200-day non-stop flight in a bird. Nat. Commun. 2013, 4, 2554. [Google Scholar] [CrossRef] [Green Version]
- Hedenström, A.; Norevik, G.; Warfvinge, K.; Andersson, A.; Bäckman, J.; Åkesson, S. Annual 10-month aerial life phase in the common swift Apus apus. Curr. Biol. 2016, 26, 3066–3070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, A.G.; Hesselberg, T.; Nesbit, R.; Goodenough, A.E. The spatial distribution and environmental triggers of ant mating flights: Using citizen-science data to reveal national patterns. Ecography 2018, 41, 877–888. [Google Scholar] [CrossRef] [Green Version]
- Boda, P.; Csabai, Z. When do beetles and bugs fly? A unified scheme for describing seasonal flight behaviour of highly dispersing primary aquatic insects. Hydrobiologia 2013, 703, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Bauer, S.; Chapman, J.W.; Reynolds, D.R.; Alves, J.A.; Dokter, A.M.; Menz, M.M.H.; Sapir, N.; Ciach, M.; Pettersson, L.B.; Kelly, J.F.; et al. From agricultural benefits to aviation safety: Realizing the potential of continent-wide radar networks. Bioscience 2017, 67, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.W.; Reynolds, D.R.; Wilson, K. Long-range seasonal migration in insects: Mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 2015, 18, 287–302. [Google Scholar] [CrossRef] [Green Version]
- Bauer, S.; Shamoun-Baranes, J.; Nilsson, C.; Farnsworth, A.; Kelly, J.F.; Reynolds, D.R.; Dokter, A.M.; Krauel, J.F.; Petterson, L.B.; Horton, K.G.; et al. The grand challenges of migration ecology that radar aeroecology can help answer. Ecography 2019, 42, 861–875. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, C.; Dokter, A.M.; Verlinden, L.; Shamoun-Baranes, J.; Schmid, B.; Desmet, P.; Bauer, S.; Chapman, J.; Alves, J.A.; Stepanian, P.M.; et al. Revealing patterns of nocturnal migration using the European weather radar network. Ecography 2019, 42, 876–886. [Google Scholar] [CrossRef] [Green Version]
- Schmid, B.; Zaugg, S.; Votier, S.C.; Chapman, J.W.; Boos, M.; Liechti, F. Size matters in quantitative radar monitoring of animal migration: Estimating monitored volume from wingbeat frequency. Ecography 2019, 42, 931–941. [Google Scholar] [CrossRef]
- Zaugg, S.; Saporta, G.; van Loon, E.; Schmaljohann, H.; Liechti, F. Automatic identification of bird targets with radar via patterns produced by wing flapping. J. R. Soc. Interface 2008, 5, 1041–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liechti, F.; Bruderer, B. Quantification of nocturnal bird migration by moonwatching: Comparison with radar and infrared observations. J. F. Ornithol. 1995, 66, 457–468. [Google Scholar]
- Schmaljohann, H.; Liechti, F.; Bächler, E.; Steuri, T.; Bruderer, B. Quantification of bird migration by radar—A detection probability problem. Ibis 2008, 150, 342–355. [Google Scholar] [CrossRef]
- Batschelet, E. Circular Statistics in Biology; Academic Press: Cambridge, MA, USA, 1981. [Google Scholar]
- Kemp, M.U.; Shamoun-Baranes, J.; van Gasteren, H.; Bouten, W.; van Loon, E.E. Can wind help explain seasonal differences in avian migration speed? J. Avian Biol. 2010, 41, 672–677. [Google Scholar] [CrossRef]
- Tschanz, P.; Pellissier, L.; Shi, X.; Liechti, F.; Schmid, B. Consistency of spatio-temporal patterns of avian migration across the Swiss lowlands. Remote Sens. Ecol. Conserv. 2020, 6, 198–211. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2017; ISBN 9781498728348. [Google Scholar]
- Killick, R.; Eckley, I.A. Changepoint: An R package for changepoint analysis. J. Stat. Softw. 2014, 58, 1–19. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Van Belle, J.; Shamoun-Baranes, J.; van Loon, E.; Bouten, W. An operational model predicting autumn bird migration intensities for flight safety. J. Appl. Ecol. 2007, 44, 864–874. [Google Scholar] [CrossRef]
- Karlsson, H.; Nilsson, C.; Bäckman, J.; Alerstam, T. Nocturnal passerine migrants fly faster in spring than in autumn: A test of the time minimization hypothesis. Anim. Behav. 2012, 83, 87–93. [Google Scholar] [CrossRef]
- Weisshaupt, N.; Dokter, A.M.; Arizaga, J.; Maruri, M. Effects of a sea barrier on large-scale migration patterns studied by a network of weather radars. Bird Study 2018, 65, 232–240. [Google Scholar] [CrossRef]
- Nilsson, C.; Dokter, A.M.; Schmid, B.; Scacco, M.; Verlinden, L.; Bäckman, J.; Haase, G.; Dell’Omo, G.; Chapman, J.W.; Leijnse, H.; et al. Field validation of radar systems for monitoring bird migration. J. Appl. Ecol. 2018, 55, 2552–2564. [Google Scholar] [CrossRef]
- Fransson, T. Timing and speed of migration in north and west European populations of sylvia warblers. J. Avian Biol. 1995, 26, 39. [Google Scholar] [CrossRef]
- Hüppop, O.; Hüppop, K. North Atlantic oscillation and timing of spring migration in birds. Proc. R. Soc. B 2003, 270, 233–240. [Google Scholar] [CrossRef]
- Erni, B.; Liechti, F.; Underhill, L.G.; Bruderer, B. Wind and rain govern the intensity of nocturnal bird migration in central Europe—A log-linear regression analysis. Ardea 2002, 90, 155–166. [Google Scholar]
- Liechti, F.; Scandolara, C.; Rubolini, D.; Ambrosini, R.; Korner-Nievergelt, F.; Hahn, S.; Lardelli, R.; Romano, M.; Caprioli, M.; Romano, A.; et al. Timing of migration and residence areas during the non-breeding period of barn swallows Hirundo rustica in relation to sex and population. J. Avian Biol. 2015, 46, 254–265. [Google Scholar] [CrossRef]
- Robinson, R.A.; Meier, C.M.; Witvliet, W.; Kéry, M.; Schaub, M. Survival varies seasonally in a migratory bird: Linkages between breeding and non-breeding periods. J. Anim. Ecol. 2020, 89, 2111–2121. [Google Scholar] [CrossRef]
- Åkesson, S.; Ilieva, M.; Karagicheva, J.; Rakhimberdiev, E.; Tomotani, B.; Helm, B. Timing avian long-distance migration: From internal clock mechanisms to global flights. Philos. Trans. R. Soc. B 2017, 372, 20160252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behr, O.; Brinkmann, R.; Hochradel, K.; Mages, J.; Korner-Nievergelt, F.; Niermann, I.; Reich, M.; Simon, R.; Weber, N.; Nagy, M. Mitigating bat mortality with turbine-specific curtailment algorithms: A model based approach. In Wind Energy and Wildlife Interactions; Springer: Berlin/Heidelberg, Germany, 2017; pp. 135–160. [Google Scholar]
- Salvarina, I.; Gravier, D.; Rothhaupt, K.O. Seasonal bat activity related to insect emergence at three temperate lakes. Ecol. Evol. 2018, 8, 3738–3750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.G. Migration and Dispersal of Insects by Flight; Methuen: London, UK, 1969. [Google Scholar]
- Stefanescu, C.; Puig-Montserrat, X.; Samraoui, B.; Izquierdo, R.; Ubach, A.; Arrizabalaga, A. Back to Africa: Autumn migration of the painted lady butterfly Vanessa cardui is timed to coincide with an increase in resource availability. Ecol. Entomol. 2017, 42, 737–747. [Google Scholar] [CrossRef]
- Kelly, J.F.; Horton, K.G.; Stepanian, P.M.; de Beurs, K.M.; Fagin, T.; Bridge, E.S.; Chilson, P.B. Novel measures of continental-scale avian migration phenology related to proximate environmental cues. Ecosphere 2016, 7, e01434. [Google Scholar] [CrossRef]
- Visser, M.E.; Both, C. Shifts in phenology due to global climate change: The need for a yardstick. Proc. R. Soc. B 2005, 272, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.H.; Dennis, R.L.H.; Croxton, P.J.; Cade, M. Increased migration of Lepidoptera linked to climate change. Eur. J. Entomol. 2007, 104, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Bale, J.S.; Hayward, S.A.L. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Schmid, B.; Tschanz, P.; Segelbacher, G.; Liechti, F. Seasonal Trends in Movement Patterns of Birds and Insects Aloft Simultaneously Recorded by Radar. Remote Sens. 2021, 13, 1839. https://doi.org/10.3390/rs13091839
Shi X, Schmid B, Tschanz P, Segelbacher G, Liechti F. Seasonal Trends in Movement Patterns of Birds and Insects Aloft Simultaneously Recorded by Radar. Remote Sensing. 2021; 13(9):1839. https://doi.org/10.3390/rs13091839
Chicago/Turabian StyleShi, Xu, Baptiste Schmid, Philippe Tschanz, Gernot Segelbacher, and Felix Liechti. 2021. "Seasonal Trends in Movement Patterns of Birds and Insects Aloft Simultaneously Recorded by Radar" Remote Sensing 13, no. 9: 1839. https://doi.org/10.3390/rs13091839
APA StyleShi, X., Schmid, B., Tschanz, P., Segelbacher, G., & Liechti, F. (2021). Seasonal Trends in Movement Patterns of Birds and Insects Aloft Simultaneously Recorded by Radar. Remote Sensing, 13(9), 1839. https://doi.org/10.3390/rs13091839