A Machine Learning Approach for Mapping Chlorophyll Fluorescence at Inland Wetlands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Site
2.2. Field Measurements
2.3. Satellite Data Acquisition
2.4. Vegetation Index Calculation
2.5. Model Establishment and Evaluation
3. Results
3.1. Analysis of Ground-Measured Chlorophyll Fluorescence
3.2. Features Selected by Algorithm for Mapping ChF
3.3. Evaluation of the Model Performance
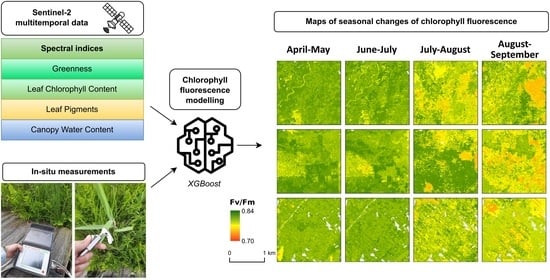
3.4. Spatial and Temporal Patterns of Chlorophyll Fluorescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krause, G.H.; Weis, E. Chlorophyll fluorescence as a tool in plant physiology. Photosynth. Res. 1984, 5, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, C. Variability and application of the chlorophyll fluorescence emission ratio red/far-red of leaves. Photosynth. Res. 2007, 92, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Michele, M.; Micol, R.; Luis, G.; Luis, A.; Roberto, C.; Jose, M. Remote sensing of solar-induced chlorophyll fluorescence: Review of methods and applications. Remote Sens. Environ. 2009, 113, 2037–2051. [Google Scholar] [CrossRef]
- Mohammed, G.H.; Colombo, R.; Middleton, E.M.; Rascher, U.; van der Tol, C.; Nedbal, L.; Goulas, Y.; Pérez-Priego, O.; Damm, A.; Meroni, M.; et al. Remote sensing of solar-induced chlorophyll fluorescence (SIF) in vegetation: 50 years of progress. Remote Sens. Environ. 2019, 231, 111177. [Google Scholar] [CrossRef]
- OS5p+ Pulse Modulated Chlorophyll Fluorometer, User Guide, OptiScience. 2022. Available online: https://www.optisci.com/ (accessed on 1 April 2023).
- Baker, N.R.; Oxborough, K. Chlorophyll Fluorescence as a Probe of Photosynthetic Productivity. In Chlorophyll a Fluorescence. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19. [Google Scholar] [CrossRef]
- Du, S.; Liu, L.; Liu, X.; Hu, J. Response of Canopy Solar-Induced Chlorophyll Fluorescence to the Absorbed Photosynthetically Active Radiation Absorbed by Chlorophyll. Remote Sens. 2017, 9, 911. [Google Scholar] [CrossRef]
- Jia, M.; Li, D.; Colombo, R.; Wang, Y.; Wang, X.; Cheng, T.; Zhu, Y.; Yao, X.; Xu, C.; Ouer, G.; et al. Quantifying Chlorophyll Fluorescence Parameters from Hyperspectral Reflectance at the Leaf Scale under Various Nitrogen Treatment Regimes in Winter Wheat. Remote Sens. 2019, 11, 2838. [Google Scholar] [CrossRef]
- Joiner, J.; Guanter, L.; Lindstrot, R.; Voigt, M.; Vasilkov, A.; Middleton, E.; Huemmrich, K.; Yoshida, Y.; Frankenberg, C. Global monitoring of terrestrial chlorophyll fluorescence from moderate-spectral-resolution near-infrared satellite measurements: Methodology, simulations, and application to GOME-2. Atmos. Meas. Tech. 2013, 6, 3883–3930. [Google Scholar] [CrossRef]
- Duveiller, G.; Filipponi, F.; Walther, S.; Köhler, P.; Frankenberg, C.; Guanter, L.; Cescatti, A. A spatially downscaled sun-induced fluorescence global product for enhanced monitoring of vegetation productivity. Earth Syst. Sci. Data 2020, 12, 1101–1116. [Google Scholar] [CrossRef]
- Li, X.; Xiao, J.; He, B.; Arain, M.A.; Beringer, J.; Desai, A.R.; Emmel, C.; Hollinger, D.Y.; Krasnova, A.; Mammarella, I.; et al. Solar-induced chlorophyll fluorescence is strongly correlated with terrestrial photosynthesis for a wide variety of biomes: First global analysis based on OCO-2 and flux tower observations. Glob. Change Biol. 2018, 24, 3990–4008. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.K.E.; Huemmrich, K.F.; Middleton, E.M.; Ward, L.A.; Julitta, T.; Daughtry, C.S.T.; Burkart, A.; Russ, A.L.; Kustas, W.P. Diurnal and Seasonal Variations in Chlorophyll Fluorescence Associated with Photosynthesis at Leaf and Canopy Scales. Remote Sens. 2019, 11, 488. [Google Scholar] [CrossRef]
- Radosław, G.; Maciej, B. Remote sensing techniques to assess chlorophyll fluorescence in support of crop monitoring in Poland. Misc. Geogr. 2021, 25, 226–237. [Google Scholar] [CrossRef]
- Guo, M.; Li, J.; Li, J.; Zhong, C.; Zhou, F. Solar-Induced Chlorophyll Fluorescence Trends and Mechanisms in Different Ecosystems in Northeastern China. Remote Sens. 2022, 14, 1329. [Google Scholar] [CrossRef]
- Kritten, L.; Preusker, R.; Fischer, J. A New Retrieval of Sun-Induced Chlorophyll Fluorescence in Water from Ocean Colour Measurements Applied on OLCI L-1b and L-2. Remote Sens. 2020, 12, 3949. [Google Scholar] [CrossRef]
- Sinha, S.K.; Padalia, H.; Patel, N.R.; Chauhan, P. Estimation of Seasonal Sun-Induced Fluorescence Dynamics of Indian Tropical Deciduous Forests using SCOPE and Sentinel-2 MSI. Int. J. Appl. Earth Obs. Geoinf. 2020, 91, 102155. [Google Scholar] [CrossRef]
- Buman, B.; Hueni, A.; Colombo, R.; Cogliati, S.; Celesti, M.; Julitta, T.; Burkart, A.; Siegmann, B.; Rascher, U.; Drusch, M.; et al. Towards consistent assessments of in situ radiometric measurements for the validation of fluorescence satellite missions. Remote Sens. Environ. 2022, 274, 112984. [Google Scholar] [CrossRef]
- Poddar, S.; Chacko, N.; Swain, D. Estimation of Chlorophyll-a in Northern Coastal Bay of Bengal Using Landsat-8 OLI and Sentinel-2 MSI Sensors. Front. Mar. Sci. 2019, 6, 598. [Google Scholar] [CrossRef]
- Smith, B.; Pahlevan, N.; Schalles, J.; Ruberg, S.; Errera, R.; Ma, R.; Giardino, C.; Bresciani, M.; Barbosa, C.; Moore, T.; et al. A Chlorophyll-a Algorithm for Landsat-8 Based on Mixture Density Networks. Front. Remote Sens. 2021, 1, 623678. [Google Scholar] [CrossRef]
- Young, K.; Taeho, K.; Jihoon, S.; Dae-Seong, L.; Park, Y.-S.; Yeji, K.; Yoonkyung, C. Validity evaluation of a machine-learning model for chlorophyll a retrieval using Sentinel-2 from inland and coastal waters. Ecol. Indic. 2022, 137, 108737. [Google Scholar] [CrossRef]
- Shi, X.; Gu, L.; Jiang, T.; Zheng, X.; Dong, W.; Tao, Z. Retrieval of Chlorophyll-a Concentrations Using Sentinel-2 MSI Imagery in Lake Chagan Based on Assessments with Machine Learning Models. Remote Sens. 2022, 14, 4924. [Google Scholar] [CrossRef]
- Wassen, M.; Okruszko, T.; Kardel, I.; Chormański, J.; Świątek, D.; Mioduszewski, W.; Bleuten, W.; Querner, E.; El Kahloun, M.; Batellan, O.; et al. Eco-Hydrological Functioning of Biebrza Wetlands: Lessons for the Conservation and Restoration of Deteriorated Wetlands. Ecol. Stud. 2006, 191, 285–310. [Google Scholar]
- Ignacy, K.; Dorota, S.; Jaroslaw, C.; Tomasz, O.; Martin, W. Water management decision support system for biebrza national park. Environ. Prot. Eng. 2009, 35, 173–180. [Google Scholar]
- Budzyńska, M.; Dąbrowska-Zielińska, K.; Turlej, K.; Małek, I.; Bartold, M. Monitoring of the Biebrza Wetlands using remote sensing methods. Water-Environ.-Rural. Areas 2011, 11, 39–64. [Google Scholar]
- Dabrowska-Zielinska, K.; Budzynska, M.; Tomaszewska, M.; Bartold, M.; Gatkowska, M.; Malek, I.; Turlej, K.; Napiorkowska, M. Monitoring Wetlands Ecosystems Using ALOS PALSAR (L-Band, HV) Supplemented by Optical Data: A Case Study of Biebrza Wetlands in Northeast Poland. Remote Sens. 2014, 6, 1605–1633. [Google Scholar] [CrossRef]
- Dabrowska-Zielinska, K.; Budzynska, M.; Tomaszewska, M.; Malinska, A.; Gatkowska, M.; Bartold, M.; Malek, I. Assessment of Carbon Flux and Soil Moisture in Wetlands Applying Sentinel-1 Data. Remote Sens. 2016, 8, 756. [Google Scholar] [CrossRef]
- Okruszko, H. Wetlands of the Biebrza valley, Their Value and Future Management; Polish Academy of Sciences: Warsaw, Poland, 1990. [Google Scholar]
- Pawłowski, B.; Medwecka-Kornaś, A.; Kornaś, J. Review of Terrestrial and Fresh-water Plant Communities. In International Series of Monographs in Pure and Applied Biology; The Vegetation of Poland; Szafer, W., Ed.; Wiley-Blackwell Publishing Ltd.: Hoboken, NJ, USA, 1966; Volume 9. [Google Scholar] [CrossRef]
- Okruszko, T.; Chormański, J.; Mirosław-Świątek, D.; Gregorczyk, M. Hydrological Characteristics of Swamp Communities, the Biebrza River (NE Poland) Case Study; Environmental Hydraulics Taylor & Francis Group: London, UK, 2010; pp. 407–412. [Google Scholar]
- Tomasz, B.; Martin, W.; Jan, S.; Jaroslaw, C.; Stefan, I.; Okke, B.; Tomasz, O. Wetlands in flux: Looking for the drivers in a central European case. Wetl. Ecol. Manag. 2018, 26, 849–863. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta 1975, 376, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Kycko, M.; Romanowska, E.; Zagajewski, B. Lead-Induced Changes in Fluorescence and Spectral Characteristics of Pea Leaves. Remote Sens. 2019, 11, 1885. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Available online: https://philippgaertner.github.io/2020/08/percent-cloud-cover/ (accessed on 28 February 2023).
- Arnon, K.; Yoram, K.; Lorraine, R.; Andrew, W. AFRI—Aerosol free vegetation index. Remote Sens. Environ. 2001, 71, 10–21. [Google Scholar]
- Kaufman, Y.J.; Tanré, D. Atmospherically Resistant Vegetation Index (ARVI) for EOS-MODIS. IEEE Trans. Geosci. Remote Sens. 1992, 30, 261–270. [Google Scholar] [CrossRef]
- Perry, C., Jr.; Lautenschlager, L.F. Functional Equivalence of Spectral Vegetation Indices. Remote Sens. Environ. 1984, 14, 169–182. [Google Scholar] [CrossRef]
- Huete, A.R.; Justice, C.; Liu, H. Development of vegetation soil indices for, M.O.D.I.S.-E.O.S. Remote Sens. Environ. 1994, 49, 224–234. [Google Scholar] [CrossRef]
- Sripada, R.P.; Heiniger, R.W.; White, J.G.; Weisz, R. Aerial color infrared photography for determining late-season nitrogen requirements in corn. Agron. J. 2005, 97, 1443–1451. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Walker, C.N.; Angus, J.F. Estimating The Nitrogen Status Of Crops Using A Digital Camera. Field Crops Res. 2010, 118, 221–227. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative estimation of chlorophyll a using reflectance spectra: Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Jurgens, C. The modified normalized difference vegetation index (mNDVI) a new index to determine frost damages in agriculture based on Landsat TM data. Int. J. Remote Sens. 1997, 18, 3583–3594. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with E.R.T.S. In Proceedings of the 3rd Earth Resources Technology Satellite Symposium, Washington, DC, USA, 10–14 December 1973; pp. 309–317. [Google Scholar]
- Roujean, J.; Breon, F. Estimating PAR Absorbed by Vegetation from Bidirectional Reflectance Measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Edward, B.; Clarke, T.R.; Richards, S.E.; Paul, C.; Julio, H.; Kostrzewski, M.; Peter, W.; Christopher, C.; Riley, E.; Thompson, T.L. Coincident detection of crop water stress, nitrogen status, and canopy density using ground based multispectral data. In Proceedings of the the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Graciela, M. Vegetation indices derived from high-resolution airborne videography for precision crop management. Int. J. Remote Sens. 2003, 24, 2855–2877. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus Hippocastanum L. and Acer platanoides L. Leaves. J. Plant. Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Sims, D.; Gamon, J. Relationships Between Leaf Pigment Content and Spectral Reflectance Across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Huete, A.R. A soil adjusted vegetation index SAVI. Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- LLymburner, P.J.; Beggs, C.R. Jacobson, Estimation of canopy-average surface-specific leaf area using Landsat TM data. Photogramm. Eng. Remote Sens. 2000, 66, 183–191. [Google Scholar]
- Jordan, C.F. Derivation of leaf area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Pearson, R.L.; Miller, L.D. Remote mapping of standing crop biomass for estimation of the productivity of the shortgrass prairie. In Proceedings of the Eighth International Symposium on Remote Sensing of Environment, Ann Arbor, MI, USA, 2–6 October 1972; Environmental Research Institute of Michigan: Ann Arbor, MI, USA, 1972; pp. 1357–1381. [Google Scholar]
- Frederic, B.; Guyot, G.; Bégué, A.; Maurel, P.; Podaire, A. Complementarity of middle-infrared with visible and near-infrared reflectance for monitoring wheat canopies. Remote Sens. Environ. 1988, 26, 213–225. [Google Scholar] [CrossRef]
- Frampton, W.; Dash, J.; Watmough, G.; Milton, E. Evaluating the capabilities of Sentinel-2 for quantitative estimation of biophysical variables in vegetation. ISPRS J. Photogramm. Remote Sens. 2013, 82, 83–92. [Google Scholar] [CrossRef]
- Elshikha, D.E.; Barnes, E.; Clarke, T.; Hunsaker, D.; Haberland, J.; Pinter, P.; Waller, P.; Thompson, T.L. Remote Sensing of Cotton Nitrogen Status Using the Canopy Chlorophyll Content Index (CCCI). Trans. ASABE 2008, 51, 73–82. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant. Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Datt, B. Remote Sensing of Water Content in Eucalyptus Leaves. J. Plant. Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; Brown de Colstoun, E.; McMurtrey, J.E. Estimating corn leaf chlorophyll concentration for leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Haboudane, D.; Tremblay, N.; Miller, J.R.; Vigneault, P. Remote estimation of crop chlorophyll content using spectral indices derived from hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2008, 46, 423–437. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.; Chivkunova, O. Optical Properties and Nondestructive Estimation of Anthocyanin Content in Plant Leaves. Photochem. Photobiol. 2001, 71, 38–45. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Matín, P.; Cachorro, V.; Gonzáles, M.R.; de Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Chivkunova, O.; Solovchenko, A.; Sokolova, S.; Merzlyak, M.; Reshetnikova, I. Reflectance Spectral Features and Detection of Superficial Scald–induced Browning in Storing Apple Fruit. Pap. Nat. Resour. 2001, 2, 267. [Google Scholar]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially located platform and aerial photography for documentation of grazing impacts on wheat. Geocarto Int. 2001, 16, 65–70. [Google Scholar] [CrossRef]
- Nidamanuri, R.; Garg, P.K.; Sanjay, G.; Vinay, D. Estimation of leaf total chlorophyll and nitrogen concentrations using hyperspectral satellite imagery. J. Agric. Sci. 2008, 146, 65–75. [Google Scholar]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Josep, P.; Baret, F.; Iolanda, F. Semi-Empirical Indices to Assess Carotenoids/Chlorophyll—A Ratio from Leaf Spectral Reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Ceccato, P.; Gobron, N.; Flasse, S.; Pinty, B.; Tarantola, S. Designing a spectral index to estimate vegetation water content from remote sensing data: Part 1 Theoretical approach. Remote Sens. Environ. 2002, 82, 188–197. [Google Scholar] [CrossRef]
- Hunt, E.R.; Rock, B.N. Detection of changes in leaf water content using near- and middle-infrared reflectances. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar]
- Hardisky, M.A.; Klemas, V.; Smart, R.M. The influence of soil salinity, growth form, and leaf moisture on the spectral reflectances of Spartina alterniflora canopies. Photogramm. Eng. Remote Sens. 1983, 49, 77–83. [Google Scholar]
- Gao, B. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.J. NMDI: A normalized multi-band drought index for monitoring soil and vegetation moisture with satellite remote sensing. Geophys. Res. Lett. 2007, 34, L20405. [Google Scholar] [CrossRef]
- Rasmus, F.; Inge, S. Derivation of a Shortwave Infrared Water Stress Index From MODIS Near- and Shortwave Infrared Data in a Semiarid Environment. Remote Sens. Environ. 2003, 87, 111–121. [Google Scholar]
- Wen, L.; Hughes, M. Coastal Wetland Mapping Using Ensemble Learning Algorithms: A Comparative Study of Bagging, Boosting and Stacking Techniques. Remote Sens. 2020, 12, 1683. [Google Scholar] [CrossRef]
- Jafarzadeh, H.; Mahdianpari, M.; Gill, E.W.; Brisco, B.; Mohammadimanesh, F. Remote Sensing and Machine Learning Tools to Support Wetland Monitoring: A Meta-Analysis of Three Decades of Research. Remote Sens. 2022, 14, 6104. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, T.; Wang, X.; Hu, Y. Chlorophyll Fluorescence Imaging Combined with Active Oxygen Metabolism for Classification of Similar Diseases in Cucumber Plants. Agronomy 2023, 13, 700. [Google Scholar] [CrossRef]
- Dąbrowska-Zielińska, K.; Misiura, K.; Malińska, A.; Gurdak, R.; Bartold, M.; Kluczek, M.; Grzybowski, P. Modelling Net Ecosystem Exchange in the Biebrza Wetlands using satellite and meteorological data. Misc. Geogr. 2022, 26, 215–226. [Google Scholar] [CrossRef]
- Dąbrowska-Zielińska, K.; Misiura, K.; Malińska, A.; Gurdak, R.; Grzybowski, P.; Bartold, M.; Kluczek, M. Spatiotemporal estimation of gross primary production for terrestrial wetlands using satellite and field data. Remote Sens. Appl. Soc. Environ. 2022, 27, 100786. [Google Scholar] [CrossRef]
- Šimanauskienė, R.; Linkevičienė, R.; Bartold, M.; Dąbrowska-Zielińska, K.; Slavinskienė, G.; Veteikis, D.; Taminskas, J. Peatland degradation: The relationship between raised bog hydrology and normalized difference vegetation index. Ecohydrology 2019, 12, e2159. [Google Scholar] [CrossRef]
- Amoros-Lopez, J.; Vila-Frances, J.; Gomez-Chova, L.; Alonso, L.; Guanter, L.; del Valle-Tascon, S.; Calpe, J.; Moreno, J. Remote sensing of chlorophyll fluorescence for estimation of stress in vegetation. recommendations for future missions. In Proceedings of the 2007 IEEE International Geoscience and Remote Sensing Symposium, Barcelona, Spain, 23–28 July 2007; pp. 3769–3772. [Google Scholar] [CrossRef]
- Batelaan, O.; Okruszko, T.; Mirosław-Świątek, D.; Sylwia, S.-W.; Jaroslaw, C.; Martin, W.; Van Loon, A.; Penning, W. Biebrza wetland research: Required science for sustainable management. In Proceedings of the 15th Annual Sustainable Development Research Conference, Utrecht, The Netherlands, 5–8 July 2009. [Google Scholar]
- Sucholas, J.; Molnár, Z.; Łuczaj, Ł.; Poschlod, P. Local traditional ecological knowledge about hay management practices in wetlands of the Biebrza Valley, Poland. J. Ethnobiol. Ethnomedicine 2022, 18, 9. [Google Scholar] [CrossRef]
- Jing, X.; Zou, Q.; Yan, J.; Dong, Y.; Li, B. Remote Sensing Monitoring of Winter Wheat Stripe Rust Based on mRMR-XGBoost Algorithm. Remote Sens. 2022, 14, 756. [Google Scholar] [CrossRef]
- Shepherd, J.D.; Schindler, J.; Dymond, J.R. Automated Mosaicking of Sentinel-2 Satellite Imagery. Remote Sens. 2020, 12, 3680. [Google Scholar] [CrossRef]
- Cui, Z.; Kerekes, J.P. Impact of Wavelength Shift in Relative Spectral Response at High Angles of Incidence in Landsat-8 Operational Land Imager and Future Landsat Design Concepts. IEEE Trans. Geosci. Remote Sens. 2018, 56, 5873–5883. [Google Scholar] [CrossRef]
- Swiatek, D.; Szporak-Nasilowska, S.; Chormanski, J.; Okruszko, T. Hydrodynamic model of the Lower Biebrza River flow—A tool for assessing the hydrologic vulnerability of a floodplain to management practices. Ecohydrol. Hydrobiol. 2008, 8, 331–337. [Google Scholar]
- Grygoruk, M.; Okruszko, T. Do Water Management and Climate-Adapted Management of Wetlands Interfere in Practice? Lessons from the Biebrza Valley, Poland. In Wetlands and Water Framework Directive. GeoPlanet: Earth and Planetary Sciences; Ignar, S., Grygoruk, M., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]









| Name | Start Date | End Date | No. of Sentinel-2 Acquisition Dates Used for Mosaic |
|---|---|---|---|
| April–May | 25 April 2022 | 12 May 2022 | 7 |
| June–July | 20 June 2022 | 7 July 2022 | 9 |
| July–August | 20 July 2022 | 4 August 2022 | 7 |
| August–September | 20 August 2022 | 8 September 2022 | 10 |
| September–October | 20 September 2022 | 28 October 2022 | 14 |
| Application | Abbreviation | Name | Equation | Citation |
|---|---|---|---|---|
| Spectral Indices of Greenness | AFRI1600 | Aerosol Free Vegetation Index 1600 | [37] | |
| ARVI | Atmospherically Resistant Vegetation Index | [38] | ||
| CTVI | Corrected Transformed Vegetation Index | [39] | ||
| EVI | Enhanced Vegetation Index | [40] | ||
| GDVI | Green Difference Vegetation Index | [41] | ||
| GI | Greenness Index | [42] | ||
| GNDVI | Green Normalized Difference Vegetation Index | [43] | ||
| mNDVI | Modified NDVI | [44] | ||
| NDVI | Normalized Difference Vegetation Index | [45] | ||
| rNDVI | Renormalized Difference Vegetation Index | [46] | ||
| NDRE | Normalized Difference NIR / Red Edge | [47] | ||
| PPR | Normalized Difference 550/450 Plant Pigment Ratio | [48] | ||
| PVR | Normalized Difference 550/650 Photosynthetic Vigour Ratio | [48] | ||
| RENDVI | Red Edge Normalized Difference Vegetation Index | [49,50] | ||
| SAVI | Soil Adjusted Vegetation Index | [51] | ||
| SLAVI | Specific Leaf Area Vegetation Index | [52] | ||
| SR | Simple Ratio 842/665 | [53,54] | ||
| SRT | Simple Ratio 1610/2190 | [55] | ||
| S2REP | Sentinel-2 Red-Edge Position Index | [56] | ||
| Leaf Chlorophyll Content | CCCI | Canopy Chlorophyll Content Index | [57] | |
| CVI | Red-edge-band Chlorophyll Index | [58] | ||
| IRECI | Inverted Red-edge Chlorophyll Index | [56] | ||
| LCI | Leaf Chlorophyll Index | [59] | ||
| MCARI | Modified Chlorophyll Absorption in Reflectance Index | [60] | ||
| TCARI | Transformed Chlorophyll Absorption Ratio | [61] | ||
| TCI | Triangular Chlorophyll Index | [62] | ||
| Leaf Pigments | ARI | Anthocyanin Reflectance Index | [63] | |
| BGI | Blue Green Pigment Index | [64] | ||
| BRI | Browning Reflectance Index | [65] | ||
| CI | Coloration Index | [58] | ||
| GLI | Green Leaf Index | [66] | ||
| PBI | Plant Biochemical Index | [67] | ||
| PSRI | Plant Senescence Reflectance Index | [68] | ||
| SIPI | Structure Insensitive Pigment Index | [69] | ||
| Canopy Water Content | GVMI | Global Vegetation Moisture Index | [70] | |
| MSI | Moisture Stress Index | [71] | ||
| NDII | Normalized Difference Infrared Index | [72] | ||
| NDWI | Normalized Difference Water Index | [73] | ||
| NMDI | Normalized Multi-band Drought Index | [74] | ||
| SIWSI | Shortwave Infrared Water Stress Index | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartold, M.; Kluczek, M. A Machine Learning Approach for Mapping Chlorophyll Fluorescence at Inland Wetlands. Remote Sens. 2023, 15, 2392. https://doi.org/10.3390/rs15092392
Bartold M, Kluczek M. A Machine Learning Approach for Mapping Chlorophyll Fluorescence at Inland Wetlands. Remote Sensing. 2023; 15(9):2392. https://doi.org/10.3390/rs15092392
Chicago/Turabian StyleBartold, Maciej, and Marcin Kluczek. 2023. "A Machine Learning Approach for Mapping Chlorophyll Fluorescence at Inland Wetlands" Remote Sensing 15, no. 9: 2392. https://doi.org/10.3390/rs15092392
APA StyleBartold, M., & Kluczek, M. (2023). A Machine Learning Approach for Mapping Chlorophyll Fluorescence at Inland Wetlands. Remote Sensing, 15(9), 2392. https://doi.org/10.3390/rs15092392