Using Ordinary Digital Cameras in Place of Near-Infrared Sensors to Derive Vegetation Indices for Phenology Studies of High Arctic Vegetation
Abstract
:1. Introduction
- Can different greenness indices derived from images captured by near remote sensing digital cameras be used to reliably monitor the phenological cycle of separate High Arctic plant species/groups, and which index best represents such changes?
- How well correlated was the best performing greenness index, derived from digital camera images, with NDVI data?
- Can different near remote sensing NDVI devices reliably monitor the greenness of separate High Arctic plant species/groups, and how well correlated were the data gathered by these sensors?
2. Materials and Methods
2.1. Study Area and Equipment Set-Up
2.2. Sensor Descriptions and Monitoring Setup
3. Data Preparation
4. Statistical Analyses
5. Results
5.1. Comparison of Different RGB Greenness Indices
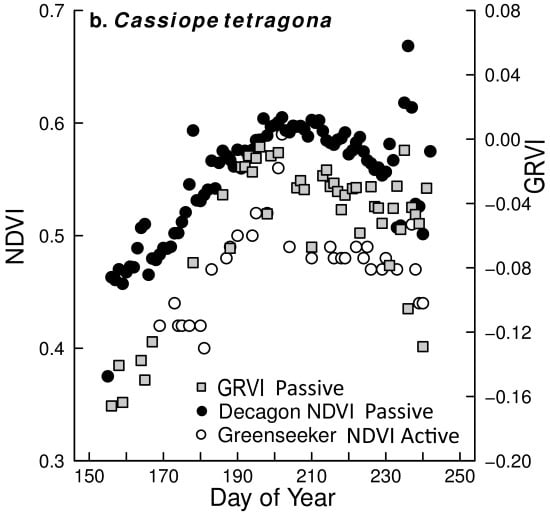
5.2. Capturing Changes in Plant Greenness during the Growing Season
5.3. Comparison of Different NDVI Sensors
6. Discussion
7. Conclusions
- Comparing the Decagon and Greenseeker NDVI correlation for the three vegetation types equipped with Decagon sensors, the r was 0.80, 0.78 and 0.70 respectively.
- However, the (passive) Decagon sensor had higher correlations with GRVI than the (active) Greenseeker (Decagon 0.88, 0.8 and 0.84, Greenseeker 0.56, 0.69 and 0.52).
- Among the three RGB greenness indices derived from the digital cameras, the GRVI had the highest and most significant correlation of NDVI with both (passive) Decagon and (active) Greenseeker derived NDVI. Still, correlations of 2G_RBi and Channel G% with the Decagon were high in all vegetation types (ranging from 0.72 to 0.86), but less so with the Greenseeker where one (2G_RBi) or two (Channel G%) correlations were insignificant.
- The greater variability in NDVI measurement and poorer performance of (active) Greenseeker compared to (passive) Decagon indicate the need for more precise and rigid procedures for field measurement; the Greenseeker should be placed in a fixed position, aspect, slope and elevation for each field point measured.
- Correlations between NDVI values derived from (passive) Decagon/(active) Greenseeker and the RGB derived vegetation indices obtained from the digital cameras, especially the GRVI, indicate that both approaches capture similar vegetation attributes and are capable of being used to monitor phenological change in different plant species/groups. Thus, both NDVI sensors and RGB cameras could be used to establish an High Arctic near remote sensing monitoring network with the aim of validating high spatial resolution satellite based products.
- Inexpensive RGB cameras offer a more affordable alternative to the more specialized and expensive NDVI sensors for monitoring plant greenness. This may enable a greater spatial coverage for field campaigns.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morisette, J.T.; Richardson, A.D.; Knapp, A.K.; Fisher, J.I.; Graham, E.A.; Abatzoglou, J.; Wilson, B.E.; Breshears, D.D.; Henebry, G.M.; Hanes, J.M.; et al. Tracking the rhythm of the seasons in the face of global change: Phenological research in the 21st century. Front. Ecol. Environ. 2009, 7, 253–260. [Google Scholar] [CrossRef]
- Sparks, T.H.; Carey, P.D. The responses of species to climate over 2 centuries—An analysis of the Marsham phenological record, 1736–1947. J. Ecol. 1995, 83, 321–329. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kubler, K.; Bissolli, P.; Braslavska, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Callaghan, T.V.; Tweedie, C.E.; Akerman, J.; Andrews, C.; Bergstedt, J.; Butler, M.G.; Christensen, T.R.; Cooley, D.; Dahlberg, U.; Danby, R.K.; et al. Multi-decadal changes in tundra environments and ecosystems: Synthesis of the international polar year-back to the future project (IPY-BTF). Ambio 2011, 40, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.S.; Schneider, D.P.; McKay, N.P.; Ammann, C.M.; Bradley, R.S.; Briffa, K.R.; Miller, G.H.; Otto-Bliesner, B.L.; Overpeck, J.T.; Vinther, B.M.; et al. Recent warming reverses long-term arctic cooling. Science 2009, 325, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Brock, C.A.; Cozic, J.; Bahreini, R.; Froyd, K.D.; Middlebrook, A.M.; McComiskey, A.; Brioude, J.; Cooper, O.R.; Stohl, A.; Aikin, K.C.; et al. Characteristics, sources, and transport of aerosols measured in spring 2008 during the aerosol, radiation, and cloud processes affecting arctic climate (ARCPAC) project. Atmos. Chem. Phys. 2011, 11, 2423–2453. [Google Scholar] [CrossRef] [Green Version]
- Vettoretti, G.; D’Orgeville, M.; Peltier, W.R.; Stastna, M. Polar climate instability and climate teleconnections from the arctic to the midlatitudes and tropics. J. Clim. 2009, 22, 3513–3539. [Google Scholar] [CrossRef]
- Bjorkman, A.D.; Elmendorf, S.C.; Beamish, A.L.; Velland, M.; Henry, G.H.R. Contrasting effects of warming and increased snowfall on Arctic tundra plant phenology over the past two decades. Glob. Chang. Biol. 2015, 21, 4651–4661. [Google Scholar] [CrossRef] [PubMed]
- Semenchuk, P.R.; Gillespie, M.A.K.; Rumpf, S.B.; Baggesen, N.S.; Elberling, B.; Cooper, E.J. High Arctic plant phenology is determined by snowmelt patterns but duration of phenological periods is fixed: An example of periodicity. Environ. Res. Lett. 2016, in press. [Google Scholar]
- Oberbauer, S.F.; Elmendorf, S.C.; Troxler, T.G.; Hollister, R.D.; Rocha, A.V.; Bret-Harte, M.S.; Dawes, M.A.; Fosaa, A.M.; Henry, G.H.R.; Hoye, T.T.; et al. Phenological response of tundra plants to background climate variation tested using the international tundra experiment. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Prevéy, J.; Vellend, M.; Ruger, N.; Hollister, R.; Bjorkman, A.; Meyers-Smith, I.; Elmendorf, S.; Clark, K.; Cooper, E.J.; Elberling, B.; et al. Greater temperature sensitivity of plant phenology at colder sites: Implications for convergence across northern latitudes. Glob. Chang. Biol. 2016. submitted. [Google Scholar]
- Kerr, J.T.; Ostrovsky, M. From space to species: Ecological applications for remote sensing. Trends Ecol. Evol. 2003, 18, 299–305. [Google Scholar] [CrossRef]
- Turner, W.; Spector, S.; Gardiner, N.; Fladeland, M.; Sterling, E.; Steininger, M. Remote sensing for biodiversity science and conservation. Trends Ecol. Evol. 2003, 18, 306–314. [Google Scholar] [CrossRef]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Myneni, R.B.; Hall, F.G.; Sellers, P.J.; Marshak, A.L. The interpretation of spectral vegetation indexes. IEEE Trans. Geosci. Remote Sens. 1995, 33, 481–486. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Stow, D.A.; Hope, A.; McGuire, D.; Verbyla, D.; Gamon, J.; Huemmrich, F.; Houston, S.; Racine, C.; Sturm, M.; Tape, K.; et al. Remote sensing of vegetation and land-cover change in arctic tundra ecosystems. Remote Sens. Environ. 2004, 89, 281–308. [Google Scholar] [CrossRef]
- Pettorelli, N.; Pettorelli, N. Normalized Difference Vegetation Index; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A.R. A review of vegetation indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Richardson, A.D.; Jenkins, J.P.; Braswell, B.H.; Hollinger, D.Y.; Ollinger, S.V.; Smith, M.L. Use of digital webcam images to track spring green-up in a deciduous broadleaf forest. Oecologia 2007, 152, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ide, R.; Oguma, H. Use of digital cameras for phenological observations. Ecol. Inform. 2010, 5, 339–347. [Google Scholar] [CrossRef]
- Eastman, R.; Warren, S.G. Arctic cloud changes from surface and satellite observations. J. Clim. 2010, 23, 4233–4242. [Google Scholar] [CrossRef]
- Hope, A.S.; Stow, D.A. Shortwave reflectance properties of arctic tundra landscapes. In Landscape Function and Disturbance in Arctic Tundra; Reynolds, J.F., Tenhunen, J.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 155–164. [Google Scholar]
- Beck, P.S.A.; Atzberger, C.; Høgda, K.A.; Johansen, B.; Skidmore, A.K. Improved monitoring of vegetation dynamics at very high latitudes: A new method using MODIS NDVI. Remote Sens. Environ. 2006, 100, 321–334. [Google Scholar] [CrossRef]
- Toomey, M.; Friedl, M.A.; Frolking, S.; Hufkens, K.; Klosterman, S.; Sonnentag, O.; Baldocchi, D.D.; Bernacchi, C.J.; Biraud, S.C.; Bohrer, G.; et al. Greenness indices from digital cameras predict the timing and seasonal dynamics of canopy-scale photosynthesis. Ecol. Appl. 2015, 25, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Motohka, T.; Nasahara, K.N.; Oguma, H.; Tsuchida, S. Applicability of green-red vegetation index for remote sensing of vegetation phenology. Remote Sens. 2010, 2, 2369–2387. [Google Scholar] [CrossRef]
- Migliavacca, M.; Galvagno, M.; Cremonese, E.; Rossini, M.; Meroni, M.; Sonnentag, O.; Cogliati, S.; Manca, G.; Diotri, F.; Busetto, L.; et al. Using digital repeat photography and eddy covariance data to model grassland phenology and photosynthetic CO2 uptake. Agric. For. Meteorol. 2011, 151, 1325–1337. [Google Scholar] [CrossRef]
- Sonnentag, O.; Hufkens, K.; Teshera-Sterne, C.; Young, A.M.; Friedl, M.; Braswell, B.H.; Milliman, T.; O’Keefe, J.; Richardson, A.D. Digital repeat photography for phenological research in forest ecosystems. Agric. For. Meteorol. 2012, 152, 159–177. [Google Scholar] [CrossRef]
- Buus-Hinkler, J.; Hansen, B.U.; Tamstorf, M.P.; Pedersen, S.B. Snow-vegetation relations in a high arctic ecosystem: Inter-annual variability inferred from new monitoring and modeling concepts. Remote Sens. Environ. 2006, 105, 237–247. [Google Scholar] [CrossRef]
- Westergaard-Nielsen, A.; Lund, M.; Hansen, B.U.; Tamstorf, M.P. Camera derived vegetation greenness index as proxy for gross primary production in a low arctic wetland area. ISPRS J. Photogramm. Remote Sens. 2013, 86, 89–99. [Google Scholar] [CrossRef]
- Johansen, B.E.; Karlsen, S.R.; Tømmervik, H. Vegetation mapping of Svalbard utilising Landsat TM/ETM+ data. Polar Rec. 2012, 48, 47–63. [Google Scholar] [CrossRef]
- Rønning, O.I. The Flora of Svalbard; Norwegian Polar Institute: Tromsø, Norway, 1996. [Google Scholar]
- Spectral Reflectance Sensor (SRS). Available online: https://www.decagon.com/en/canopy/canopy-measurements/spectral-reflectance-sensor-srs/ (accessed on 3 March 2016).
- Gamon, J.A.; Kovalchuck, O.; Wong, C.Y.S.; Harris, A.; Garrity, S.R. Monitoring seasonal and diurnal changes in photosynthetic pigments with automated PRI and NDVI sensors. Biogeosciences 2015, 12, 4149–4159. [Google Scholar] [CrossRef]
- GreenSeeker Handheld Crop Sensor. Available online: http://www.trimble.com/Agriculture/gs-handheld.aspx (accessed on 3 March 2016).
- Inman, D.; Khosla, R.; Reich, R.M.; Westfall, D.G. Active remote sensing and grain yield in irrigated maize. Precis. Agric. 2007, 8, 241–252. [Google Scholar] [CrossRef]
- Brinno Timelapse Cameras. Available online: http://www.brinno.com/html/GWC-intro.html (accessed on 3 March 2016).
- Cuddeback. Available online: http://cuddeback.com/index.aspx (accessed on 3 March 2016).
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Bernard, E.; Friedt, J.M.; Tolle, F.; Griselin, M.; Martin, G.; Laffly, D.; Marlin, C. Monitoring seasonal snow dynamics using ground based high resolution photography. ISPRS J. Photogramm. Remote Sens. 2013, 75, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Hinkler, J.; Orbaek, J.B.; Hansen, B.U. Detection of spatial, temporal, and spectral surface changes in the Ny-Ålesund area 79 degrees N, Svalbard, using a low cost multispectral camera in combination with spectroradiometer measurements. Phys. Chem. Earth 2003, 28, 1229–1239. [Google Scholar] [CrossRef]
- Hinkler, J.; Pedersen, S.B.; Rasch, M.; Hansen, B.U. Automatic snow cover monitoring at high temporal and spatial resolution, using images taken by a standard digital camera. Int. J. Remote Sens. 2002, 23, 4669–4682. [Google Scholar] [CrossRef]
- Eiken, T.; Sund, M. Photogrammetric methods applied to Svalbard glaciers: Accuracies and challenges. Polar Res. 2012, 31, 16–20. [Google Scholar] [CrossRef]
- Tape, K.D.; Gustine, D.D. Capturing migration phenology of terrestrial wildlife using camera traps. Bioscience 2014, 64, 117–124. [Google Scholar] [CrossRef]
- Inoue, T.; Nagai, S.; Kobayashi, H.; Koizumi, H. Utilization of ground-based digital photography for the evaluation of seasonal changes in the aboveground green biomass and foliage phenology in a grassland ecosystem. Ecol. Inform. 2015, 25, 1–9. [Google Scholar] [CrossRef]
- Thuestad, A.E.; Tømmervik, H.; Solbø, S.A.; Barlindhaug, S.; Flyen, A.C.; Myrvoll, E.R.; Johansen, B. Monitoring cultural heritage environments in Svalbard: Smeerenburg, a whaling station on Amsterdam island. EARSeL eProc. 2015, 14, 37–50. [Google Scholar]
- Richardson, A.D.; Braswell, B.H.; Hollinger, D.Y.; Jenkins, J.P.; Ollinger, S.V. Near-surface remote sensing of spatial and temporal variation in canopy phenology. Ecol. Appl. 2009, 19, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Van der Wal, R.; Stien, A. High-arctic plants like it hot: A long-term investigation of between-year variability in plant biomass. Ecology 2014, 95, 3414–3427. [Google Scholar] [CrossRef]
- Mizunuma, T.; Wilkinson, M.; Eaton, E.L.; Mencuccini, M.; Morison, J.I.L.; Grace, J. The relationship between carbon dioxide uptake and canopy colour from two camera systems in a deciduous forest in Southern England. Funct. Ecol. 2013, 27, 196–207. [Google Scholar] [CrossRef]
- Von Bueren, S.K.; Burkart, A.; Hueni, A.; Rascher, U.; Tuohy, M.P.; Yule, I.J. Deploying four optical UAV-based sensors over grassland: Challenges and limitations. Biogeosciences 2015, 12, 163–175. [Google Scholar] [CrossRef]
- Pushkareva, E.; Pessi, I.S.; Wilmotte, A.; Elster, J. Cyanobacterial community composition in arctic soil crusts at different stages of development. FEMS Microbiol. Ecol. 2015, 91, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Lee, Y.J.; Yang, C.M.; Change, K.W.; Shen, Y. A simple spectral index using reflectance of 735 nm to assess nitrogen status of rice canopy. Agron. J. 2008, 100, 205–212. [Google Scholar] [CrossRef]
- Rumpf, S.B.; Semenchuk, P.R.; Dullinger, S.; Cooper, E.J. Idiosyncratic responses of high arctic plants to changing snow regimes. PLoS ONE 2014, 9, 10–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, E.J.; Dullinger, S.; Semenchuk, P. Late snowmelt delays plant development and results in lower reproductive success in the high arctic. Plant Sci. 2011, 180, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callaghan, T.V.; Carlsson, B.A.; Tyler, N.J.C. Historical records of climate-related growth in Cassiope tetragona from the arctic. J. Ecol. 1989, 77, 823–837. [Google Scholar] [CrossRef]
- Semenchuk, P.R.; Elberling, B.; Cooper, E.J. Snow cover and extreme winter warming events control flower abundance of some, but not all species in high arctic Svalbard. Ecol. Evol. 2013, 3, 2586–2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arndal, M.F.; Illeris, L.; Michelsen, A.; Albert, K.; Tamstorf, M.; Hansen, B.U. Seasonal variation in gross ecosystem production, plant biomass, and carbon and nitrogen pools in five high arctic vegetation types. Arct. Antarct. Alpine Res. 2009, 41, 164–173. [Google Scholar] [CrossRef]
- Abbandonato, H.; Semenchuk, P.R.; Elberling, B.; Cooper, E.J. Snowmelt timing and soil temperature as important drivers for autumn senescence in High Arctic Svalbard. Environ. Res. Lett. 2016, in press. [Google Scholar]
- Wang, Q.; Tenhunen, J.; Dinh, N.Q.; Reichstein, M.; Vesala, T.; Keronen, P. Similarities in ground- and satellite-based NDVI time series and their relationship to physiological activity of a scots pine forest in finland. Remote Sens. Environ. 2004, 93, 225–237. [Google Scholar] [CrossRef]
- Van der Wal, R.; Hessen, D.O. Analogous aquatic and terrestrial food webs in the high arctic: The structuring force of a harsh climate. Perspect. Plant Ecol. Evol. Syst. 2009, 11, 231–240. [Google Scholar] [CrossRef]
- Sakamoto, T.; Gitelson, A.A.; Nguy-Robertson, A.L.; Arkebauer, T.J.; Wardlow, B.D.; Suyker, A.E.; Verma, S.B.; Shibayama, M. An alternative method using digital cameras for continuous monitoring of crop status. Agric. For. Meteorol. 2012, 154, 113–126. [Google Scholar] [CrossRef]
- Fitzgerald, G.J. Characterizing vegetation indices derived from active and passive sensors. Int. J. Remote Sens. 2010, 31, 4335–4348. [Google Scholar] [CrossRef]
- Yao, X.F.; Yao, X.; Jia, W.Q.; Tian, Y.C.; Ni, J.; Cao, W.X.; Zhu, Y. Comparison and intercalibration of vegetation indices from different sensors for monitoring above-ground plant nitrogen uptake in winter wheat. Sensors 2013, 13, 3109–3130. [Google Scholar] [CrossRef] [PubMed]
- Steven, M.D.; Malthus, T.J.; Baret, F.; Xu, H.; Chopping, M.J. Intercalibration of vegetation indices from different sensor systems. Remote Sens. Environ. 2003, 88, 412–422. [Google Scholar] [CrossRef]





| Vegetation | NDVI Sensor | GRVI | 2G_RBi | Channel G % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t | p | r | t | p | r | t | p | r | ||
| Graminoid/Salix polaris | D | t82 = 16.55 | <0.001 | 0.88 | t80 = 9.11 | <0.001 | 0.72 | t81 = 9.76 | <0.001 | 0.74 |
| G | t33 = 3.73 | 0.001 | 0.56 | t30 = 2.62 | 0.014 | 0.44 | t30 = 2.42 | 0.023 | 0.42 | |
| Cassiope tetragona | D | t76 = 11.45 | <0.001 | 0.8 | t78 = 14.52 | <0.001 | 0.86 | t75 = 12.34 | <0.001 | 0.82 |
| G | t28 = 4.87 | <0.001 | 0.69 | t29 = 3.58 | 0.001 | 0.57 | t27 = 3.01 | 0.006 | 0.52 | |
| Luzula spp. | G | t15 = 0.4 | 0.698 | 0.11 | t15 = 3.01 | 0.01 | 0.64 | t15 = 3.29 | 0.006 | 0.67 |
| D. octopetala/S. polaris | G | t31 = 3.26 | 0.003 | 0.52 | t29 = 3.01 | 0.006 | 0.5 | t31 = 1.81 | 0.08 | 0.32 |
| C. tetragona/D. octopetala | D | t81 = 13.76 | <0.001 | 0.84 | t79 = 12.05 | <0.001 | 0.81 | t80 = 10.54 | <0.001 | 0.77 |
| G | t32 = 3.35 | 0.002 | 0.52 | t32 = 1.0 | 0.325 | 0.18 | t32 = 0.66 | 0.513 | 0.12 | |
| Graminoid/bryophyte | G | t30 = 6.87 | <0.001 | 0.79 | t30 = 9.44 | <0.001 | 0.87 | t30 = 6.59 | <0.001 | 0.78 |
| Vegetation | Decagon NDVI | Greenseeker NDVI | GRVI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | R2 | F | p | R2 | F | p | R2 | |
| Graminoid/Salix polaris | F2,89 = 46.3 | <0.001 | 0.52 | F2,33 = 8.34 | <0.001 | 0.36 | F2,82 = 113.53 | <0.001 | 0.74 |
| Cassiope tetragona | F2,89 = 33.57 | <0.001 | 0.44 | F2,33 = 17.73 | <0.001 | 0.54 | F2,76 = 198.13 | <0.001 | 0.84 |
| Luzula spp. | - | - | - | F2,34 = 21.51 | <0.001 | 0.58 | F2,35 = 14.55 | 0.009 | 0.48 |
| D. octopetala/S. polaris | - | - | - | F2,34 = 20.4 | <0.001 | 0.57 | F2,79 = 45.86 | <0.001 | 0.55 |
| C. tetragona/D. octopetala | F2,88 = 72.7 | <0.001 | 0.63 | F2,34 = 9.14 | 0.062 | 0.37 | F2,81 = 214.37 | <0.001 | 0.85 |
| Graminoid/bryophyte | - | - | - | F2,31 = 22.32 | <0.001 | 0.61 | F2,69 = 204.36 | <0.001 | 0.86 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, H.B.; Nilsen, L.; Tømmervik, H.; Karlsen, S.R.; Nagai, S.; Cooper, E.J. Using Ordinary Digital Cameras in Place of Near-Infrared Sensors to Derive Vegetation Indices for Phenology Studies of High Arctic Vegetation. Remote Sens. 2016, 8, 847. https://doi.org/10.3390/rs8100847
Anderson HB, Nilsen L, Tømmervik H, Karlsen SR, Nagai S, Cooper EJ. Using Ordinary Digital Cameras in Place of Near-Infrared Sensors to Derive Vegetation Indices for Phenology Studies of High Arctic Vegetation. Remote Sensing. 2016; 8(10):847. https://doi.org/10.3390/rs8100847
Chicago/Turabian StyleAnderson, Helen B., Lennart Nilsen, Hans Tømmervik, Stein Rune Karlsen, Shin Nagai, and Elisabeth J. Cooper. 2016. "Using Ordinary Digital Cameras in Place of Near-Infrared Sensors to Derive Vegetation Indices for Phenology Studies of High Arctic Vegetation" Remote Sensing 8, no. 10: 847. https://doi.org/10.3390/rs8100847
APA StyleAnderson, H. B., Nilsen, L., Tømmervik, H., Karlsen, S. R., Nagai, S., & Cooper, E. J. (2016). Using Ordinary Digital Cameras in Place of Near-Infrared Sensors to Derive Vegetation Indices for Phenology Studies of High Arctic Vegetation. Remote Sensing, 8(10), 847. https://doi.org/10.3390/rs8100847