Multitemporal Monitoring of Plant Area Index in the Valencia Rice District with PocketLAI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Field Campaign
2.2. Spatial Sampling Strategy
2.3. Effective Plant Area Index (PAIeff)
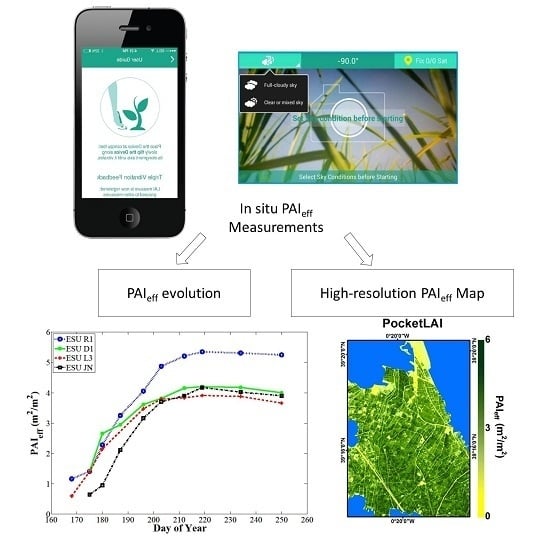
2.4. PocketLAI
2.5. Digital Hemispherical Photography (DHP)
2.6. LAI-2000 Plant Canopy Analyzer
2.7. Complementary Field Data
2.7.1. Phenology
2.7.2. Chlorophyll Content
2.8. Transfer Function for High-Resolution PAIeff Mapping
3. Results
3.1. On the Temporal Evolution of the PAIeff Field Measurements
3.2. On the Ancillary Data: Phenology and Leaf Chlorophyll Content
3.3. On the PAIeff Measuring Instruments and Maps Comparison
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Whelan, B.; McBratney, A. The “Null Hypothesis” of precision agriculture management. Precis. Agric. 2000, 2, 265–279. [Google Scholar] [CrossRef]
- Stafford, J.V. Implementing precision agriculture in the 21st Century. J. Agr. Eeng. Res. 2000, 76, 267–275. [Google Scholar] [CrossRef]
- Atzberger, C. Advances in remote sensing of agriculture: Context description, existing operational monitoring systems and major information needs. Remote Sens. 2013, 5, 949–981. [Google Scholar] [CrossRef]
- Colgan, M.S.; Baldeck, C.A.; Féret, J.B.; Asner, G.P. Mapping savanna tree species at ecosystem scales using support vector machine classification and BRDF correction on airborne hyperspectral and LiDAR data. Remote Sens. 2012, 4, 3462–3480. [Google Scholar] [CrossRef]
- D’Oleire-Oltmanns, S.; Marzolff, I.; Peter, K.D.; Ries, J.B. Unmanned Aerial Vehicle (UAV) for monitoring soil erosion in Morocco. Remote Sens. 2012, 4, 3390–3416. [Google Scholar] [CrossRef]
- Marshall, M.; Thenkabail, P. Developing in situ non-destructive estimates of crop biomass to address issues of scale in remote sensing. Remote Sens. 2015, 7, 808–835. [Google Scholar] [CrossRef]
- Son, N.T.; Chen, C.F.; Chen, C.R.; Duc, H.N.; Chang, L.Y. A phenology-based classification of time-series MODIS data for rice crop monitoring in Mekong Delta, Vietnam. Remote Sens. 2013, 6, 135–156. [Google Scholar] [CrossRef]
- López-Sánchez, J.; Vicente-Guijalba, F.; Ballester-Berman, J.; Cloude, S. Polarimetric response of rice fields at C-Band: Analysis and phenology retrieval. IEEE Trans. Geosci. Remote Sens. 2014, 52, 2977–2993. [Google Scholar] [CrossRef]
- Nelson, A.; Setiyono, T.; Rala, A.; Quicho, E.; Raviz, J.; Abonete, P.; Maunahan, A.; Garcia, C.; Bhatti, H.; Villano, L.; et al. Towards an operational SAR-Based rice monitoring system in ASIA: Examples from 13 demonstration sites across asia in the RIICE project. Remote Sens. 2014, 6, 10773–10812. [Google Scholar] [CrossRef]
- Karila, K.; Nevalainen, O.; Krooks, A.; Karjalainen, M.; Kaasalainen, S. Monitoring changes in rice cultivated area from SAR and optical satellite images in Ben Tre and Tra Vinh Provinces in Mekong Delta, Vietnam. Remote Sens. 2014, 6, 4090–4108. [Google Scholar] [CrossRef]
- Chen, J.; Black, T. Defining leaf area index for non-flat leaves. Plant Cell Environ. 1992, 15, 421–429. [Google Scholar] [CrossRef]
- Vuolo, F.; Neugebauer, N.; Bolognesi, S.F.; Atzberger, C.; D’Urso, G. Estimation of Leaf Area Index using DEIMOS-1 data: Application and transferability of a semi-empirical relationship between two agricultural areas. Remote Sens. 2013, 5, 1274–1291. [Google Scholar] [CrossRef]
- Richter, K.; Hank, T.B.; Vuolo, F.; Mauser, W.; D’Urso, G. Optimal exploitation of the Sentinel-2 spectral capabilities for crop leaf area index mapping. Remote Sens. 2012, 4, 561–582. [Google Scholar] [CrossRef] [Green Version]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- GCOS. Systematic Observation Requirements for Satellite-Based Productsfor Climate, 2011 Update, Supplemental Details to the Satellite-Based Com-ponent of the Implementation Plan for the Global Observing System forClimate in Support of the UNFCCC (2010 Update). World Meteorological Organization (WMO): Geneva, Switzerland, 2011. [Google Scholar]
- Breda, N.J.J. Ground-based measurements of leaf area index: A review of methods, instruments and current controversies. J. Exp. Bot. 2003, 54, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Baret, F.; Smith, G.; Jonckheere, I.; Coppin, P. Review of methods for in situ leaf area index (LAI) determination: Part II. Estimation of LAI, errors and sampling. Agric. For. Meteorol. 2004, 121, 37–53. [Google Scholar] [CrossRef]
- Confalonieri, R.; Stroppiana, D.; Boschetti, M.; Gusberti, D.; Bocchi, S.; Acutis, M. Analysis of rice sample size variability due to development stage, nitrogen fertilization, sowing technique and variety using the visual jackknife. F. Crop. Res. 2006, 97, 135–141. [Google Scholar] [CrossRef]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of methods for in situ leaf area index determination: Part I. Theories, sensors and hemispherical photography. Agric. For. Meteorol. 2004, 121, 19–35. [Google Scholar] [CrossRef]
- Warren-Wilson, J. Estimation of foliage denseness and foliage angle by inclined point quadrats. Aust. J. Bot. 1963, 11, 95–105. [Google Scholar] [CrossRef]
- Ross, J. The Radiation Regime and Architecture of Plant Stands; Springer Science & Business Media: Hague, The Netherlands, 1981. [Google Scholar]
- Garrigues, S.; Shabanov, N.; Swanson, K.; Morisette, J.; Baret, F.; Myneni, R. Intercomparison and sensitivity analysis of Leaf Area Index retrievals from LAI-2000, AccuPAR, and digital hemispherical photography over croplands. Agric. For. Meteorol. 2008, 148, 1193–1209. [Google Scholar] [CrossRef]
- Verger, A.; Martínez, B.; Camacho, F.; García-Haro, F.J. Accuracy assessment of fraction of vegetation cover and leaf area index estimates from pragmatic methods in a cropland area. Int. J. Remote Sens. 2009, 30, 3849–3849. [Google Scholar] [CrossRef]
- Confalonieri, R.; Foi, M.; Casa, R.; Aquaro, S.; Tona, E.; Peterle, M.; Boldini, A.; Carli, G.D.; Ferrari, A.; Finotto, G.; et al. Development of an app for estimating leaf area index using a smartphone. Trueness and precision determination and comparison with other indirect methods. Comput. Electron. Agric. 2013, 96, 67–74. [Google Scholar] [CrossRef]
- Francone, C.; Pagani, V.; Foi, M.; Cappelli, G.; Confalonieri, R. Comparison of leaf area index estimates by ceptometer and PocketLAI smart app in canopies with different structures. F. Crop. Res. 2014, 155, 38–41. [Google Scholar] [CrossRef]
- Frazer, G.W.; Trofymow, J.A.; Lertzman, K.P. A Method for Estimating Canopy Openness, Effective Leaf Area Index, and Photosynthetically Active Photon Flux Density using Hemispherical Photography and Computerized Image Analysis Techniques; Technical report BC-X-373; Pacific Forestry Centre: Victoria, BC, Canada, 1997. [Google Scholar]
- Chen, J.; Black, T.; Adams, R. Evaluation of hemispherical photography for determining plant area index and geometry of a forest stand. Agric. For. Meteorol. 1991, 56, 129–143. [Google Scholar] [CrossRef]
- Demarez, V.; Duthoit, S.; Baret, F.; Weiss, M.; Dedieu, G. Estimation of leaf area and clumping indexes of crops with hemispherical photographs. Agric. For. Meteorol. 2008, 148, 644–655. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Li, W.; Wei, S.; Jiang, C. Seasonal variation of leaf area index (LAI) over paddy rice fields in NE China: Intercomparison of destructive sampling, LAI-2200, digital hemispherical photography (DHP), and AccuPAR methods. Agric. For. Meteorol. 2014, 198–199, 126–141. [Google Scholar] [CrossRef]
- Baret, F.; de Solan, B.; López-Lozano, R.; Ma, K.; Weiss, M. GAI estimates of row crops from downward looking digital photos taken perpendicular to rows at 57.5° zenith angle: Theoretical considerations based on 3D architecture models and application to wheat crops. Agric. For. Meteorol. 2010, 150, 1393–1401. [Google Scholar] [CrossRef]
- Lancashire, P.D.; Bleiholder, H.; Boom, T.V.D.; Langelüddeke, P.; Strauss, R.; Weber, E.; Witzengerger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- López-Sánchez, J.; Vicente-Guijalba, F.; Ballester-Berman, J.; Cloude, S. Influence of incidence angle on the coherent copolar polarimetric response of rice at X-Band. IEEE Geosci. Remote Sens. Lett. 2015, 12, 249–253. [Google Scholar] [CrossRef]
- Evans, J. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecolog. 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Peng, Y.; Gitelson, A.A. Remote estimation of gross primary productivity in soybean and maize based on total crop chlorophyll content. Remote Sens. Environ. 2012, 117, 440–448. [Google Scholar] [CrossRef]
- Clevers, J.; Kooistra, L. Using hyperspectral remote sensing data for retrieving canopy Chlorophyll and nitrogen content. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 574–583. [Google Scholar] [CrossRef]
- Delegido, J.; Wittenberghe, S.V.; Verrelst, J.; Ortiz, V.; Veroustraete, F.; Valcke, R.; Samson, R.; Rivera, J.P.; Tenjo, C.; Moreno, J. Chlorophyll content mapping of urban vegetation in the city of Valencia based on the hyperspectral NAOC index. Ecol. Indic. 2014, 40, 34–42. [Google Scholar] [CrossRef]
- Vincini, M.; Amaducci, S.; Frazzi, E. Empirical estimation of leaf Chlorophyll density in winter wheat canopies using Sentinel-2 spectral resolution. IEEE Trans. Geosci. Remote Sens. 2014, 52, 3220–3235. [Google Scholar] [CrossRef]
- Jin, X.; Li, Z.; Feng, H.; Xu, X.; Yang, G. Newly combined spectral indices to improve estimation of total leaf Chlorophyll content in cotton. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 4589–4600. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, G.; Zheng, K. Modeling leaf image, Chlorophyll fluorescence, reflectance from SPAD readings. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 4368–4373. [Google Scholar] [CrossRef]
- Mu, X.; Huang, S.; Ren, H.; Yan, G.; Song, W.; Ruan, G. Validating GEOV1 fractional vegetation cover derived from coarse-resolution remote sensing images over croplands. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 439–446. [Google Scholar] [CrossRef]
- Camacho, F.; Cernicharo, J.; Lacaze, R.; Baret, F.; Weiss, M. GEOV1: LAI, FAPAR essential climate variables and FCOVER global time series capitalizing over existing products. Part 2: Validation and intercomparison with reference products. Remote Sens. Environ. 2013, 137, 310–329. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, J.; Tang, S.; Li, G.; Guo, Z. Inter-Comparison and validation of the FY-3A/MERSI LAI product over mainland China. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 458–468. [Google Scholar] [CrossRef]
- Fernandes, R.; Plummer, S.; Nightingale, J.; Baret, F.; Camacho, F.; Fang, H.; Garrigues, S.; Gobron, N.; Lang, M.; Lacaze, R.; et al. Global leaf area index product validation good practices. Version 2.0. In Best Practice for Satellite-Derived Land Product Validation: Land Product Validation Subgroup (WGCV/CEOS); Schaepman-Strub, G., Román, M., Nickeson, J., Eds.; NASA: Greenbelt, MD, USA, 2014; p. 76. [Google Scholar]
- Martínez, B.; García-Haro, F.; de Coca, F.C. Derivation of high-resolution leaf area index maps in support of validation activities: Application to the cropland Barrax site. Agric. For. Meteorol. 2009, 149, 130–145. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F.; Garrigues, S.; Lacaze, R. LAI and fAPAR CYCLOPES global products derived from VEGETATION. Part 2: Validation and comparison with MODIS collection 4 products. Remote Sens. Environ. 2007, 110, 317–331. [Google Scholar] [CrossRef]
- Cohen, W.B.; Justice, C.O. Validating MODIS terrestrial ecology products: Linking in situ and satellite measurements. Remote Sens. Environ. 1999, 70, 1–3. [Google Scholar] [CrossRef]
- Nilson, T. A theoretical analysis of the frequency of gaps in plant stands. Agric. Meteorol. 1971, 8, 25–38. [Google Scholar] [CrossRef]
- Lemeur, R.; Blad, B.L. Plant modification for more efficient water use a critical review of light models for estimating the shortwave radiation regime of plant canopies. Agric. Meteorol. 1974, 14, 255–286. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F. CAN-EYE V6.313 User Manual; INRA: Avignon, France, 2010. [Google Scholar]
- Miller, J. A formula for average foliage density. Aust. J. Bot. 1967, 15, 141–144. [Google Scholar] [CrossRef]
- Campos-Taberner, M.; García-Haro, F.; Moreno, A.; Gilabert, M.; Martínez, B.; Sánchez-Ruiz, S.; Camps-Valls, G. Development of an earth observation processing chain for crop bio-physical parameters at local scale. In Proceedings of the 2015 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Milan, Italy, 26–31 July 2015; pp. 29–32.
- Asner, G.P.; Scurlock, J.M.O.; A. Hicke, J. Global synthesis of leaf area index observations: implications for ecological and remote sensing studies. Global Ecol. Biogeogr. 2003, 12, 191–205. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Hunt, E.R., Jr.; Goins, L.D.; Ustin, S.L.; Vanderbilt, V.C.; Jackson, T.J. Vegetation water content during SMEX04 from ground data and Landsat 5 Thematic Mapper imagery. Remote Sens. Environ. 2008, 112, 350–362. [Google Scholar] [CrossRef]







| Description | Principal Stage | BBCH | |
|---|---|---|---|
| Vegetative | Germination | 0 | 0–9 |
| Leaf development | 1 | 10–19 | |
| Tillering | 2 | 20–29 | |
| Stem elongation | 3 | 30–39 | |
| Booting | 4 | 40–49 | |
| Reproductive | Emergence, heading | 5 | 50–59 |
| Flowering, anthesis | 6 | 60–69 | |
| Maturation | Fruit development | 7 | 70–79 |
| Ripening | 8 | 80–89 | |
| Senescence | 9 | 90–99 | |
| Instrument | RMSE | MAE | |ME| | R2 |
|---|---|---|---|---|
| DHP | 0.67 | 0.64 | 0.61 | 0.94 |
| LAI-2000 | 0.35 | 0.33 | 0.29 | 0.98 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Taberner, M.; García-Haro, F.J.; Confalonieri, R.; Martínez, B.; Moreno, Á.; Sánchez-Ruiz, S.; Gilabert, M.A.; Camacho, F.; Boschetti, M.; Busetto, L. Multitemporal Monitoring of Plant Area Index in the Valencia Rice District with PocketLAI. Remote Sens. 2016, 8, 202. https://doi.org/10.3390/rs8030202
Campos-Taberner M, García-Haro FJ, Confalonieri R, Martínez B, Moreno Á, Sánchez-Ruiz S, Gilabert MA, Camacho F, Boschetti M, Busetto L. Multitemporal Monitoring of Plant Area Index in the Valencia Rice District with PocketLAI. Remote Sensing. 2016; 8(3):202. https://doi.org/10.3390/rs8030202
Chicago/Turabian StyleCampos-Taberner, Manuel, Franciso Javier García-Haro, Roberto Confalonieri, Beatriz Martínez, Álvaro Moreno, Sergio Sánchez-Ruiz, María Amparo Gilabert, Fernando Camacho, Mirco Boschetti, and Lorenzo Busetto. 2016. "Multitemporal Monitoring of Plant Area Index in the Valencia Rice District with PocketLAI" Remote Sensing 8, no. 3: 202. https://doi.org/10.3390/rs8030202
APA StyleCampos-Taberner, M., García-Haro, F. J., Confalonieri, R., Martínez, B., Moreno, Á., Sánchez-Ruiz, S., Gilabert, M. A., Camacho, F., Boschetti, M., & Busetto, L. (2016). Multitemporal Monitoring of Plant Area Index in the Valencia Rice District with PocketLAI. Remote Sensing, 8(3), 202. https://doi.org/10.3390/rs8030202