Accumulation and Elimination Dynamics of the Hydroxybenzoate Saxitoxin Analogues in Mussels Mytilus galloprovincialis Exposed to the Toxic Marine Dinoflagellate Gymnodinium catenatum
Abstract
:1. Introduction
2. Results and Discussion
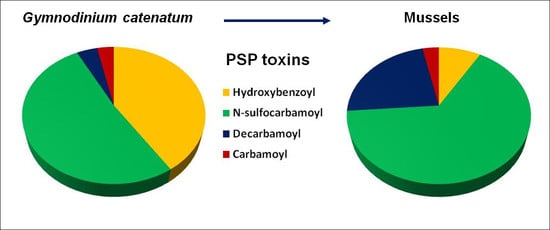
2.1. Toxin Profile of Gymnodinium Catenatum Strain Used for Mussel Exposure
2.2. Accumulation, Transformation, and Elimination of PST in Mussels
3. Conclusions
4. Materials and Methods
4.1. Gymnodinium Catenatum Cultivation
4.2. Mussels Exposure to Toxic Dinoflagellates
4.3. Determination of PST
4.3.1. Reagents
4.3.2. Determination of PST by HILIC-UPLC–MS/MS
4.3.3. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Kao, C.Y.; Nishiyama, A. Actions of saxitoxin on peripheral neuromuscular systems. J. Physiol. 1965, 180, 50–66. [Google Scholar] [PubMed]
- Ritchie, J.M.; Rogart, R.B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev. Physiol. Biochem. Pharmacol. 1977, 79, 1–49. [Google Scholar] [PubMed]
- Negri, A.P.; Stirling, D.; Quilliam, M.; Blackburn, S.; Bolch, C.; Burton, I.; Eaglesham, G.; Thomas, K.; Walter, J.; Willis, R. Three novel hydroxybenzoate saxitoxin analogues isolated from the dinoflagellate Gymnodinium catenatum. Chem. Res. Toxicol. 2003, 16, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Vale, P. Complex profiles of hydrophobic shellfish poisoning compounds in Gymnodinium catenatum identified by liquid chromatography with fluorescence detection and mass spectrometry. J. Chromatogr. A 2008, 1195, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Bustillos-Guzmán, J.J.; Band-Schmidt, C.J.; Durán-Riveroll, L.M.; Hernández-Sandoval, F.E.; López-Cortés, D.J.; Núñez-Vázquez, E.J.; Cembella, A.; Krock, B. Paralytic toxin profile of the marine dinoflagellate Gymnodinium catenatum Graham from the Mexican Pacific as revealed by LC-MS/MS. Food Addit. Cont. Part A 2015, 32, 381–394. [Google Scholar]
- Costa, P.R.; Robertson, A.; Quilliam, M.A. Toxin profile of Gymnodinium catenatum (Dinophyceae) from the Portuguese coast, as determined by Liquid Chromatography Tandem Mass Spectrometry. Mar. Drugs 2015, 13, 2046–2062. [Google Scholar] [CrossRef] [PubMed]
- Durán-Riveroll, L.M.; Krock, B.; Cembella, A.; Peralta-Cruz, J.; Bustillos-Guzmán, J.J.; Band-Schmidt, C. Characterization of Benzoyl Saxitoxin Analogs from the Toxigenic Marine Dinoflagellate Gymnodinium catenatum by Hydrophilic Interaction Liquid Ion-Chromatography-Tandem Mass Spectrometry. Nat. Prod. Chem. Res. 2017, 5, 275. [Google Scholar] [CrossRef]
- Llewellyn, L.; Negri, A.; Quilliam, M. High affinity for the rat brain sodium channel of newly discovered hydroxybenzoate saxitoxin analogues from the dinoflagellate Gymnodinium catenatum. Toxicon 2004, 43, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Durán-Riveroll, L.; Cembella, A.; Band-Schmidt, C.; Bustillos-Guzmán, J.; Correa-Basurto, J. Docking simulation of the binding interactions of saxitoxin analogs produced by the marine dinoflagellate Gymnodinium catenatum to the voltage-gated sodium channel Nav1.4. Toxins 2016, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Costa, P.R.; Porteiro, F.; Moita, T. First report of a massive bloom of Alexandrium minutum (Dinophyceae) in middle North Atlantic: A coastal lagoon in S. Jorge Island, Azores. Toxicon 2014, 90, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.R. Impact and effects of paralytic shellfish poisoning toxins derived from harmful algal blooms to marine fish. Fish Fish. 2016, 17, 226–248. [Google Scholar] [CrossRef]
- Narahashi, T.; Moore, J.W. Neuroactive agents and nerve membrane conductances. J. Gen. Physiol. 1968, 51, 93–101. [Google Scholar] [PubMed]
- Lagos, M.; Andrinolo, D. Paralytic shellfish poisoning (PSP): Toxicology and kinetics. In Seafood and Freshwater Toxins; Botana, L.M., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 203–215. [Google Scholar]
- Turner, A.D.; Lewis, A.M.; O’Neil, A.; Hatfield, R.G. Transformation of paralytic shellfish poisoning toxins in UK surf clams (Spisula solida) for targeted production of reference materials. Toxicon 2013, 65, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography—Mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; McNabb, P.S.; Harwood, D.T.; Selwood, A.I.; Boundy, M.J. Single laboratory validation of a multi-toxin UPLC-HILIC-MS/MS method for quantitation of paralytic shellfish toxins in bivalve shellfish. J. AOAC Int. 2015, 98, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Caeiro, M.F.; Costa, P.R.; Amorim, A. Gymnodinium catenatum Graham isolated from the Portuguese coast: Toxin content and genetic characterization. Harmful Algae 2015, 48, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Artigas, M.L.; Vale, P.; Gomes, S.S.; Botelho, M.J.; Rodrigues, S.M.; Amorim, A. Profiles of PSP toxins in shellfish from Portugal explained by carbamoylase activity. J. Chromatogr. A 2007, 1160, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Ogawa, N.; Takahashi, M.; Lin, H.-P.; Oshima, Y. Purification and characterization of paralytic shellfish toxin-transforming enzyme, sulfocarbamoylase I, from the Japanese bivalve Peronidia venulosa. Biochim. Biophys. Acta Proteins Proteom. 2008, 1784, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on marine biotoxins in shellfish—Saxitoxin group. EFSA J. 2009, 1019, 1–76. [Google Scholar]
- Munday, R.; Thomas, K.; Gibbs, R.; Murphy, C.; Quilliam, M.A. Acute toxicities ofsaxitoxin, neosaxitoxin, decarbamoyl saxitoxin and gonyautoxins 1&4 and 2&3to mice by various routes of administration. Toxicon 2013, 76, 77–83. [Google Scholar] [PubMed]
- Alonso, E.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Evaluation of toxicity equvalent factos of paralytic shellfish poisoning toxins in seven human sodium channels types by an automated high throughput electrophysiology system. Arch. Toxicol. 2016, 90, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Botana, L.M.; Hess, P.; Munday, R.; Natalie, A.; DeGrasse, S.L.; Feeley, M.; Suzuki, T.; van der Berg, M.; Fattori, V.; Gamarro, E.G.; et al. Derivation of toxicity equivalency factors for marine biotoxins associated with bivalve molluscs. Trends Food Sci. Technol. 2017, 59, 15–24. [Google Scholar] [CrossRef]
- Doblin, M.; Blackburn, S.; Hallegraeff, G. Comparative study of selenium requirements of three phytoplankton species: Gymnodinium catenatum, Alexandrium minutum (Dinophyta) and Chaetoceros cf. tenuissimus (Bacillariophyta). J. Plankton Res. 1999, 21, 1153–1169. [Google Scholar] [CrossRef]
- Braga, A.C.; Camacho, C.; Marques, A.; Gago-Martínez, A.; Pacheco, M.; Costa, P.R. Combined effects of warming and acidification on accumulation and elimination dynamics of paralytic shellfish toxins in mussels Mytilus galloprovincialis. Environ. Res. 2018, 164, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.R.; Botelho, M.J.; Lefebvre, K.A. Characterization of paralytic shellfish toxins in seawater and sardines (Sardina pilchardus) during blooms of Gymnodinium catenatum. Hydrobiologia 2010, 655, 89–97. [Google Scholar] [CrossRef]
- Costa, P.R.; Moita, T.; Rodrigues, S.M. Estimating the contribution of N-sulfocarbamoyl paralytic shellfish toxin analogs GTX6 and C3 + 4 to the toxicity of mussels (Mytilus galloprovincialis) over a bloom of Gymnodinium catenatum. Harmful Algae 2014, 31, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Moroño, A.; Franco, J.; Reyero, M.I. PSP detoxification kinetics in the mussel Mytilus galloprovincialis: One- and two-compartment models and the effect of some environmental variables. Mar. Ecol. Prog. Ser. 1997, 158, 165–175. [Google Scholar] [CrossRef] [Green Version]


| PSP Toxins | Toxin Concentration (fmol/cell) | Molar Fraction (%) | |
|---|---|---|---|
| N-sulfocarbamoyl | C1 | 2.0 | 10.7 |
| C2 | 5.6 | 30.1 | |
| C3 | 0.2 | 1.1 | |
| C4 | 1.4 | 7.3 | |
| GTX5 | 0.2 | 1.2 | |
| GTX6 | 0.2 | 1.2 | |
| Decarbamoyl | dcSTX | 0.3 | 1.4 |
| dcNeo | 0.2 | 1.2 | |
| dcGTX2 | 0.1 | 0.2 | |
| dcGTX3 | 0.3 | 1.4 | |
| Carbamoyl | GTX1 | 0.1 | 0.1 |
| GTX2 | 0.1 | 0.2 | |
| GTX3 | 0.5 | 2.6 | |
| GTX4 | 0.1 | 0.5 | |
| STX | <LOD | --- | |
| Neo | <LOD | --- | |
| Hydroxybenzoyl | GC1 * | 0.1 | 0.1 |
| GC2 * | 1.7 | 9.0 | |
| GC3 * | 0.4 | 2.0 | |
| GC4 * | 1.2 | 6.6 | |
| GC5 * | 3.3 | 17.5 | |
| GC6 * | 1.0 | 5.5 | |
| Toxin | GC Toxins Concentration (µmol.kg−1) | ||
|---|---|---|---|
| Day 1 | Day 5 | 24 h Elimination | |
| GC1 | 0.009 | 0.020 | 0.008 |
| GC2 | 0.158 | 0.763 | 0.412 |
| GC3 | 0.024 | 0.164 | 0.127 |
| GC4 | 0.030 | 0.082 | 0.052 |
| GC5 | 0.062 | 0.128 | 0.096 |
| GC6 | 0.118 | 0.418 | 0.513 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis Costa, P.; Braga, A.C.; Turner, A.D. Accumulation and Elimination Dynamics of the Hydroxybenzoate Saxitoxin Analogues in Mussels Mytilus galloprovincialis Exposed to the Toxic Marine Dinoflagellate Gymnodinium catenatum. Toxins 2018, 10, 428. https://doi.org/10.3390/toxins10110428
Reis Costa P, Braga AC, Turner AD. Accumulation and Elimination Dynamics of the Hydroxybenzoate Saxitoxin Analogues in Mussels Mytilus galloprovincialis Exposed to the Toxic Marine Dinoflagellate Gymnodinium catenatum. Toxins. 2018; 10(11):428. https://doi.org/10.3390/toxins10110428
Chicago/Turabian StyleReis Costa, Pedro, Ana Catarina Braga, and Andrew D. Turner. 2018. "Accumulation and Elimination Dynamics of the Hydroxybenzoate Saxitoxin Analogues in Mussels Mytilus galloprovincialis Exposed to the Toxic Marine Dinoflagellate Gymnodinium catenatum" Toxins 10, no. 11: 428. https://doi.org/10.3390/toxins10110428
APA StyleReis Costa, P., Braga, A. C., & Turner, A. D. (2018). Accumulation and Elimination Dynamics of the Hydroxybenzoate Saxitoxin Analogues in Mussels Mytilus galloprovincialis Exposed to the Toxic Marine Dinoflagellate Gymnodinium catenatum. Toxins, 10(11), 428. https://doi.org/10.3390/toxins10110428