Impact of Microcystin-LR on Liver Function Varies by Dose and Sex in Mice
Abstract
:1. Introduction
2. Results
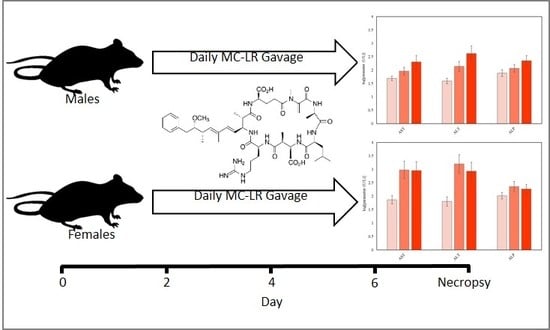
2.1. Mouse Mortality, Health, and Clinical Observations
2.2. Clinical Chemistry
2.3. Histopathology
2.4. Gap Junction Intercellular Communication
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds
4.2. Animals
4.3. Animal Husbandry
4.4. Experimental Protocol
4.5. Animal Observations
4.6. Euthanasia and Necropsy
4.7. Unexpected Deaths
4.8. Clinical Chemistry Parameters
4.9. Tissue Collection, Preservation, and Histopathology
4.10. Florescent Dye Cut-Loading
4.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United States Environmental Protection Agency (US EPA). Health Effects Support Document for the Cyanobacterial Toxin Microcystins. EPA-820R15102; US EPA: Washington, DC, USA, 2015.
- World Health Organization (WHO). Cyanobacterial Toxins: Microcystin-LR in Drinking-water (Background Document for Development of WHO Guidelines for Drinking-water Quality); Guidelines for Drinking-Water Quality: Geneva, Switzerland, 2003; p. 14. [Google Scholar]
- Backer, L.C.; Landsberg, J.H.; Miller, M.; Keel, K.; Taylor, T.K. Canine cyanotoxin poisonings in the United States (1920s–2012): Review of suspected and confirmed cases from three data sources. Toxins 2013, 5, 1597–1628. [Google Scholar] [CrossRef] [PubMed]
- Jochimsen, E.M.; Carmichael, W.W.; An, J.; Cardo, D.M.; Cookson, S.T.; Holmes, C.E.M.; Antunes, B.C.; Melo Filho, D.A.; Lyra, T.M.; Barreto, V.S.T.; et al. Liver Failure and Death After Exposure To Microcystins. N. Engl. J. Med. 1998, 339, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Azevedo, S.M.; An, J.S.; Molica, J.R.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities form cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Mrdjen, I.; Lee, J. Simple and practical on-site treatment of high microcystin levels in water using polypropylene plastic. J. Environ. Sci. Health Part A 2018, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.R.; Rosa, M.J. Microcystins removal by nanofiltration membranes. Sep. Purif. Technol. 2005, 46, 192–201. [Google Scholar] [CrossRef]
- Mrdjen, I.; Fennessy, S.; Schaal, A.; Dennis, R.; Slonczewski, J.L.; Lee, S.; Lee, J. Tile Drainage and Anthropogenic Land Use Contribute to Harmful Algal Blooms and Microbiota Shifts in Inland Water Bodies. Environ. Sci. Technol. 2018, 52, 8215–8223. [Google Scholar] [CrossRef] [PubMed]
- Bojcevska, H.; Jergil, E. Removal of cyanobacterial toxins (LPS endotoxin and microcystin) in drinking-water using the BioSand household water filter. In Minor Field Study 91; Uppsala University: Uppsala, Sweden, 2003; pp. 1–44. [Google Scholar]
- Lee, J.; Lee, S.; Jiang, X. Cyanobacterial Toxins in Freshwater and Food: Important Sources of Exposure to Humans. Annu. Rev. Food Sci. Technol. 2017, 8, 281–304. [Google Scholar] [CrossRef] [PubMed]
- Žegura, B.; Štraser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res.-Rev. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.; Louro, H.; Pinto, M.; Santos, T.; Antunes, S.; Pereira, P.; Silva, M.J. Genotoxicity of Microcystin-LR in In Vitro and In Vivo Experimental Models. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mankiewicz, J.; Walter, Z.; Tarczynska, M.; Palyvoda, O.; Wojtysiak-Staniaszczyk, M.; Zalewski, M. Genotoxicity of cyanobacterial extracts containing microcystins from polish water reservoirs as determined by SOS chromotest and comet assay. Environ. Toxicol. 2002, 17, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Fawell, J.; Mitchell, R.; Everett, D.; Hill, R. The toxicity of cyanobacterial toxins in the mouse: I. Microcystin-LR. Hum. Exp. Toxicol. 1999, 18, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Tachi, M.; Imanishi, S.Y.; Harada, K.-I. Phosphoprotein Analysis for Investigation of In Vivo Relationship Between Protein Phosphatase Inhibitory Activities and Acute Hepatotoxicity of Micorcystin-LR. Environ. Toxicol. 2007, 22, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Q.; Zhou, W.; Xu, L.; Wang, Y. Effects of chronic exposure to microcystin-LR on hepatocyte mitochondrial DNA replication in mice. Environ. Sci. Technol. 2015, 49, 4665–4672. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Makita, Y.; Nagata, S.; Tsutsumi, T.; Yoshida, F.; Sekijima, M.; Tamura, S.; Ueno, Y. Acute oral toxicity of microcystin-LR, a cyanobacterial hepatotoxin, in mice. Nat. Toxins 1997, 5, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shu, W.Q.; Qiu, Z.Q.; Chen, J.A.; Zhao, Q.; Cao, J. Protective effects of green tea polyphenols against subacute hepatotoxicity induced by microcystin-LR in mice. Environ. Toxicol. Pharmacol. 2007, 24, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Gehringer, M.M.; Shephard, E.G.; Downing, T.G.; Wiegand, C.; Neilan, B.A. An investigation into the detoxification of microcystin-LR by the glutathione pathway in Balb/c mice. Int. J. Biochem. Cell Biol. 2004, 36, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Geng, Z.; Wang, L.; Wang, X.; Geng, Z.; Zhou, Y.; Chen, Y.; Wu, J.; Han, X. Distribution of microcystin-LR to testis of male Sprague-Dawley rats. Ecotoxicology 2013, 22, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Wieckowska, A.; Zein, N.N.; Yerian, L.M.; Lopez, A.R.; McCullough, A.J.; Feldstein, A.E. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 2006, 44, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solter, P.; Liu, Z.; Guzman, R. Decreased hepatic ALT synthesis is an outcome of subchronic microcystin-LR toxicity. Toxicol. Appl. Pharmacol. 2000, 164, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.S.; Saxena, M.; Ahmad, H.; Awasthi, Y.C. Glutathione S-transferases of mouse liver: Sex-related differences in the expression of various isozymes. BBA-Gen. Subj. 1992, 1116, 137–146. [Google Scholar] [CrossRef]
- Sekijima, M.; Tsutsumi, T.; Yoshida, T.; Sekijima, M.; Tsutsumi, T.; Yoshida, T.; Harada, T.; Tashiro, F.; Chen, G.; Yu, S.Z.; Ueno, Y. Enhancement of glutathione S-transferase placental-form positive liver cell foci development by microcystin-LR in aflatoxin B1-initiated rats. Carcinogenesis 1999, 20, 161–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, G.M.; Oliveira, V.R.; Soares, R.M.; Azevedo, M.F.O.; Lima, L.M.; Barreiro, E.J.; Valenca, S.S.; Saldiva, P.H.N.; Faffe, D.S.; Zin, W.A. Can LASSBio 596 and dexamethasone treat acute lung and liver inflammation induced by microcystin-LR? Toxicon 2010, 56, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Runnegar, M.; Berndt, N.; Kaplowitz, N. Microcystin uptake and inhibition of protein phosphatases: Effects of chemoprotectants and self-inhibition in relation to known hepatic transporters. Toxicol. Appl. Pharmacol. 1995, 134, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Svircev, Z.; Baltić, V.; Gantar, M.; Juković, M.; Stojanović, D.; Baltić, M. Molecular aspects of microcystin-induced hepatotoxicity and hepatocarcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2010, 28, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, X.; Zhou, W.; Qiao, Q.; Liang, H.; Li, G.; Wang, J.; Cai, F. The Interactive Effects of Cytoskeleton Disruption and Mitochondria Dysfunction Lead to Reproductive Toxicity Induced by Microcystin-LR. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Anand, T.; Rao, P.V. Activity and gene expression profile of certain antioxidant enzymes to microcystin-LR induced oxidative stress in mice. Toxicology 2006, 20, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.M.C.; Oliveira, V.R.; Casquilho, N.V.; Araujo, A.C.P.; Soares, R.M.; Azevedo, M.F.O.; Pires, K.M.P.; Valencia, S.S.; Zin, W.A. Pulmonary and hepatic injury after sub-chronic exposure to sublethal doses of microcystin-LR. Toxicon 2016, 112, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, J.; Xiang, Z.; Jiang, P.; Du, J.; Han, X. Microcystin-LR causes sexual hormone disturbance in male rat by targeting gonadotropin-releasing hormone neurons. Toxicon 2016, 123, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Ruch, R.J.; Boucher, P.D.; Gentry, B.G.; Shewach, D.S. Gap Junctional Intercellular Communication Increases Cytotoxicity and Reduces Resistance to Hydroxyurea. J. Cancer Ther. 2014, 5, 1190–1202. [Google Scholar] [CrossRef]
- Trosko, J.E.; Ruch, J.R. Cell-Cell Communication in Carcinogenesis. Front Biosci. 1998, 3, d208–d236. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.J.; Milwid, J.M.; King, K.R.; Bohr, S.; Iracheta, A.; Li, M.; Vitalo, A.; Parekkadan, B.; Jindal, P.; Yarmush, M.L. Gap Junction Inhibition Prevents Drug-induced Liver Toxicity and Fulminant Hepatic Failure. Nat. Biotechnol. 2013, 30, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Klauning, J.E.; Ruch, R.J. Role of Intercellular Communication in Nongenotoxic Carcinogenesis. Lab. Investig. 1990, 62, 135–146. [Google Scholar]
- Trosko, J.E.; Chang, C.C. Nongenotoxic mechanisms in carcinogenesis: Role of inhibited intercellular communication. Branbury Rep. 1988, 31, 139–170. [Google Scholar]
- Ohta, T.; Nishiwaki, R.; Yatsunami, J.; Komori, A.; Suganuma, M.; Fujiki, H. Hyperphosphorylation of cytokeratins 8 and 18 by microcystin-LR, a new tumor promoter, in primary cultured rat hepatocytes. Carcinogenesis 1992, 13, 2443–2447. [Google Scholar] [CrossRef] [PubMed]
- Svircev, Z.; Krstic, S.; Miladinov-Mikov, M.; Baltic, V.; Vidovic, M. Freshwater cyanobacterial blooms and primary liver cancer epidemiological studies in Serbia. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Pant, S.C.; Vijayaraghavan, R.; Rao, P.L. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 2003, 188, 285–296. [Google Scholar] [CrossRef]
- Ruch, R.J. Intercellular communication, homeostasis, and toxicology. Toxicol. Sci. 2002, 68, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, P.R.; Lacroix, F.B.; Villa-Moruzzi, E.; Margolis, R.L. Differential subcellular localization of protein phosphatase-1 α, γ1, and δ isoforms during both interphase and mitosis in mammalian cells. J. Cell. Biol. 1998, 141, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Ruch, R.J.; Klauning, J.E. Inhibition of mouse hepatocytes intercellular communication by paraquat-generated oxygen free radicals. Toxicol. Appl. Pharmacol. 1988, 94, 427–436. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Egaas, E.; Falls, J.G.; Dauterman, W.C. A study of gender, strain and age differences in mouse liver glutathione-S-transferase. Comp. Biochem. Physiol. Part C Comp. 1995, 110, 35–40. [Google Scholar] [CrossRef]
- Sharma, R.; Ahmad, H.; Singhal, S.S.; Saxena, M.; Srivastava, S.K.; Awasthi, Y.C. Comparative studies on the effect of butylated hydroxyanisole on glutathione and glutathione S-transferases in the tissues of male and female CD-1 mice. Comp. Biochem. Physiol. C 1993, 105, 31–37. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, E.D.; Beasley, V.R. One health and cyanobacteria in freshwater systems: Animal illnesses and deaths are sentinel events for human health risks. Toxins 2015, 7, 1374–1395. [Google Scholar] [CrossRef] [PubMed]




| Clinical Parameter | Dose (µg/kg/day) | ||
|---|---|---|---|
| 0 | 3000 | 4000/5000 | |
| AST (U/L) | 49.00 ± 2.15 | 91.67 ± 8.77 a | 203.00 ± 80.17 c |
| ALT (U/L) | 39.10 ± 3.34 | 137.56 ± 22.38 b | 419.00 ± 219.08 c |
| ALP (U/L) | 77.20 ± 6.01 | 116.56 ± 8.03 | 223.67 ± 25.78 c |
| TBIL (mg/dL) | 0.15 ± 0.01 | 0.209 ± 0.01 | 0.60 ± 0.30 c |
| CHOL (mg/dL) | 157.00 ± 8.3 | 205.11 ± 12.37 b | 166.22 ± 11.03 |
| GLUC (mg/dL) | 199.90 ± 9.23 | 159.44 ± 8.33 b | 140.89 ± 9.13 c |
| Clinical Parameter | Dose (µg/kg/day) | ||
|---|---|---|---|
| 0 | 3000 | 4000/5000 | |
| AST (U/L) | 73.50 ± 11.25 | 952.40 ± 553.54 b | 891.43 ± 793.15 |
| ALT (U/L) | 63.50 ± 17.43 | 1574.50 ± 946.57 b | 857.00 ± 731.71 a |
| ALP (U/L) | 103.70 ± 5.21 | 226.70 ± 29.25 c | 184.57 ± 17.39 a |
| TBIL (mg/dL) | 0.14 ± 0.01 | 0.33 ± 0.12 | 0.23 ± 0.01 |
| CHOL (mg/dL) | 109.50 ± 2.99 | 151.70 ± 10.23 | 163.86 ± 12.97 b |
| GLUC (mg/dL) | 206.40 ± 8.62 | 136.78 ± 14.96 d | 172.14 ± 24.44 |
| Marker | Abbrev. | Maximal Fold Change Observed | |
|---|---|---|---|
| Males | Females | ||
| Aspartate aminotransferase | AST | 4.1 | 13 |
| Alanine aminotransferase | ALT | 10.7 | 24.8 |
| Alkaline Phosphatase | ALP | 2.9 | 2.2 |
| Cholesterol | CHOL | n/a | 1.5 |
| Bilirubin | TBIL | 4.1 | 2.3 |
| Mean Glucose | GLUC | (−0.8) | (−0.7) |
| Parameters | Males | Females | ||||
|---|---|---|---|---|---|---|
| Dose (µg/kg/day) | 0 | 3000 | 5000/4000 | 0 | 3000 | 5000/4000 |
| N | 10 | 10 | 10 | 10 | 10 | 10 |
| Hypertrophy | (0) a | (8) | (10) | (0) | (2) | (9) |
| Minimal | - | 6 | 1 | - | 2 | 3 |
| Mild | - | 2 | 9 | - | 0 | 6 |
| Degeneration | (0) | (5) | (10) | (0) | (4) | (7) |
| Minimal | - | 4 | 2 | - | 2 | 0 |
| Mild | - | 1 | 8 | - | 2 | 7 |
| Necrosis | (0) | (6) | (7) | (0) | (3) | (2) |
| Minimal | - | 5 | 2 | - | 1 | 1 |
| Mild | - | 0 | 3 | - | 0 | 0 |
| Moderate | - | 1 | 2 | - | 2 | 0 |
| Hemorrhage | (0) | (1) | (2) | (0) | (0) | (3) |
| Minimal | - | - | 0 | - | - | - |
| Mild | - | 1 | 2 | - | - | 3 |
| Group | MC-LR Dose (µg/kg/day) | Dose Volume (mL/kg) | Dose Concentration (µg/mL) | Number of Animals | |
|---|---|---|---|---|---|
| Males | Females | ||||
| 1 a | 2000 | 10 | 200 | 2 | 2 |
| 2 b | 2000 | 10 | 200 | 2 | 2 |
| 3 c | 2000 | 10 | 200 | 2 | 2 |
| Group | MC-LR Dose (µg/kg/day) | Dose Volume (mL/kg) | Dose Concentration (µg/mL) | Number of Animals | |
|---|---|---|---|---|---|
| Males | Females | ||||
| 1 | 0 a | 10 | 0 | 10 | 10 |
| 2 | 3000 | 10 | 300 | 10 | 10 |
| 3 | 5000/4000 b | 5000/4000 b | 500 | 10 | 10 |
| Alanine aminotransferase a Aspartate aminotransferase a Alkaline phosphatase a Gamma-glutamyltransferase a Creatine Kinase a Total bilirubin Urea nitrogen Creatinine Calcium Phosphorus Total protein | Albumin Globulin (calculated) Albumin/globulin ratio Glucose Cholesterol Triglycerides Sodium Potassium Chloride Sample Quality |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrdjen, I.; Morse, M.A.; Ruch, R.J.; Knobloch, T.J.; Choudhary, S.; Weghorst, C.M.; Lee, J. Impact of Microcystin-LR on Liver Function Varies by Dose and Sex in Mice. Toxins 2018, 10, 435. https://doi.org/10.3390/toxins10110435
Mrdjen I, Morse MA, Ruch RJ, Knobloch TJ, Choudhary S, Weghorst CM, Lee J. Impact of Microcystin-LR on Liver Function Varies by Dose and Sex in Mice. Toxins. 2018; 10(11):435. https://doi.org/10.3390/toxins10110435
Chicago/Turabian StyleMrdjen, Igor, Mark A. Morse, Randall J. Ruch, Thomas J. Knobloch, Shambhunath Choudhary, Christopher M. Weghorst, and Jiyoung Lee. 2018. "Impact of Microcystin-LR on Liver Function Varies by Dose and Sex in Mice" Toxins 10, no. 11: 435. https://doi.org/10.3390/toxins10110435
APA StyleMrdjen, I., Morse, M. A., Ruch, R. J., Knobloch, T. J., Choudhary, S., Weghorst, C. M., & Lee, J. (2018). Impact of Microcystin-LR on Liver Function Varies by Dose and Sex in Mice. Toxins, 10(11), 435. https://doi.org/10.3390/toxins10110435