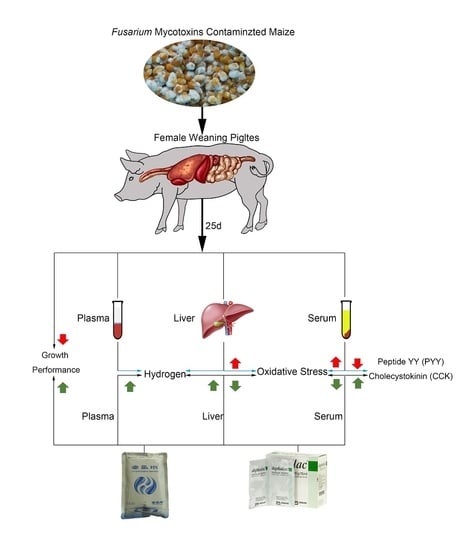
Hydrogen-Rich Water and Lactulose Protect Against Growth Suppression and Oxidative Stress in Female Piglets Fed Fusarium Toxins Contaminated Diets
Abstract
:1. Introduction
2. Results
2.1. Hydrogen Concentrations in Plasma and Liver
2.2. Growth Performance
2.3. Serum Levels of Appetite-Regulating Hormones
2.4. Oxidative and Antioxidative Status in the Serum and Liver
2.4.1. Serum Oxidant Markers and Antioxidant Capacity
2.4.2. Liver Oxidant and Antioxidant Capacity
3. Discussion
3.1. Hydrogen Concentration in Serum and Liver
3.2. Growth Performance
3.3. Serum Gut Appetite-Regulating Hormones Levels
3.4. Oxidative Stress
4. Conclusions
5. Materials and Methods
5.1. Preparation of Fusarium Contaminated Maize
5.2. Experimental Diets and Mycotoxins Analysis
5.3. Animals
5.4. Experiment Design and Sample Collection
5.5. Serum Hormones and Antioxidant Assay
5.6. Hydrogen Gas Measurement in Plasma and Liver Samples
5.7. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Item | NC 1 Diet | MC 2 Diet |
|---|---|---|
| Ingredients, % | ||
| Normal corn | 16.75 | 16.75 |
| Fusarium toxins uncontaminated corn | 44.50 | - |
| Fusarium toxins contaminated corn | - | 44.50 |
| Soybean meal | 15.79 | 15.79 |
| Extruded soybean | 10.0 | 10.0 |
| Fish meal | 5.0 | 5.0 |
| Wheat bran | 3.0 | 3.0 |
| Soybean oil | 1.74 | 1.74 |
| Vitamin and mineral premix 3 | 1.0 | 1.0 |
| Limestone powder | 0.98 | 0.98 |
| Calcium hydrogen phosphate | 0.78 | 0.78 |
| Salt | 0.37 | 0.37 |
| Lysine HCl (98%) | 0.09 | 0.09 |
| Total | 100.00 | 100.00 |
| Analyzed chemical composition 4 | ||
| DM, % | 88.96 | 88.28 |
| CP, % | 20.11 | 20.4 |
| Crude ash, % | 4.70 | 4.89 |
| Crude fiber, % | 1.71 | 1.96 |
| Ether extract, % | 8.04 | 8.65 |
| Calculated DE, 5 kcal/kg | 3400.00 | 3400.00 |
| Mycotoxin (μg/kg) | NC | MC | SEM | p-Value |
|---|---|---|---|---|
| Deoxynivalenol (DON) | 221.10 | 825.46 * | 84.34 | <0.01 |
| 3-acetyl DON | 12.12 | 212.79 * | 30.46 | <0.01 |
| 15-acetyl DON | 32.95 | 59.75 * | 4.14 | <0.01 |
| Total DON | 266.16 | 1097.99 * | 116.30 | <0.01 |
| Zearalenone (ZEN) | 9.61 | 501.56 * | 69.82 | <0.01 |
| Nivalenol (NIV) | N.D | N.D | - | - |
| T-2 | N.D | N.D | - | - |
References
- Cortinovis, C.; Pizzo, F.; Spicer, L.J.; Caloni, F. Fusarium mycotoxins: Effects on reproductive function in domestic animals—A review. Theriogenology 2013, 80, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Naehrer, K.; Rodriguess, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Danicke, S. The fusarium toxins deoxynivalenol (don) and zearalenone (zon) in animal feeding. Prev. Vet. Med. 2011, 102, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Escrivá, L.; Font, G.; Manyes, L. In vivo toxicity studies of fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Tan, B.E.; Wu, M.M.; Yin, Y.L.; Li, T.J.; Yuan, D.X.; Li, L. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: II. Intestinal morphology and function. J. Anim. Sci. 2013, 91, 4750–4756. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Danicke, S. In vivo and in vitro effects of the mycotoxins zearalenone and deoxynivalenol on different non-reproductive and reproductive organs in female pigs: A review. Food Addit. Contam. 2007, 24, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Uetsuka, K. Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int. J. Mol. Sci. 2011, 12, 5213–5237. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-J.; Zhao, Y.-Y.; Xiong, B.; Cui, X.-S.; Kim, N.-H.; Xu, Y.-X.; Sun, S.-C. Mycotoxin-containing diet causes oxidative stress in the mouse. PLoS ONE 2013, 8, e60374. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Molecular hydrogen as a novel antioxidant: Overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015, 555, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta 2012, 1820, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653–102673. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.-I.; Asoh, S.; Ishikawa, M.; Yamamoto, Y.; Ohsawa, I.; Ohta, S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. BBRC 2007, 361, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shen, W.F.; Sun, H.Y.; Fan, D.F.; Nakao, A.; Cai, J.M.; Yan, G.; Zhou, W.P.; Shen, R.X.; Yang, J.M.; et al. Hydrogen-rich saline protects against liver injury in rats with obstructive jaundice. Liver Int. 2010, 30, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Chen, X.; Lu, J.; Zhang, Y.; Sun, X.; Huang, Q.; Wang, Q. Hydrogen-rich saline improves nonalcoholic fatty liver disease by alleviating oxidative stress and activating hepatic pparalpha and ppargamma. Mol. Med. Rep. 2017, 15, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zuo, Q.; Hai, Y.; Sun, X.J. Lactulose: An indirect antioxidant ameliorating inflammatory bowel disease by increasing hydrogen production. Med. Hypotheses 2011, 76, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Chen, X.; Shi, J.; Shi, D.; Ye, Z.; Liu, W.; Li, M.; Wang, Q.; Kang, Z.; Bi, H.; et al. Lactulose ameliorates cerebral ischemia-reperfusion injury in rats by inducing hydrogen by activating nrf2 expression. Free Radic. Biol. Med. 2013, 65, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhai, X.; Shi, J.; Liu, W.W.; Tao, H.; Sun, X.; Kang, Z. Lactulose mediates suppression of dextran sodium sulfate-induced colon inflammation by increasing hydrogen production. Dig. Dis. Sci. 2013, 58, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.; Hong, Y.; Wang, Q.; Liu, X.; Li, D. Lactulose mediates suppression of dextran sulfate sodium-induced colon inflammation. J. Med. Coll. PLA 2013, 28, 65–79. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, W.; Zhang, R.; Ruan, X.; Ren, P.; Lu, B. Lactulose accelerates liver regeneration in rats by inducing hydrogen. J. Surg. Res. 2015, 195, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhou, X.; Dai, Q.; Fan, Y.; Huang, X. Hydrogen-rich saline ameliorates lung injury associated with cecal ligation and puncture-induced sepsis in rats. Exp. Mol. Pathol. 2015, 98, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Ordaz, A.A.; Gonzalez-Ortiz, G.; La Ragione, R.M.; Woodward, M.J.; Collins, J.W.; Perez, J.F.; Martin-Orue, S.M. Lactulose and lactobacillus plantarum, a potential complementary synbiotic to control postweaning colibacillosis in piglets. Appl. Environ. Microbiol. 2014, 80, 4879–4886. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kurokawa, R.; Fujino, M.; Hirano, S.; Sato, B.; Li, X.K. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci. Rep. 2014, 4, 5485. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Sano, M.; Ohsawa, I.; Shinmura, K.; Tamaki, K.; Kimura, K.; Endo, J.; Katayama, T.; Kawamura, A.; Kohsaka, S.; et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2008, 373, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.F.; Zheng, X.F.; Cai, J.M.; You, X.M.; Deng, X.M.; Zhang, J.H.; Jiang, L.; Sun, X.J. Hydrogen-rich saline reduces lung injury induced by intestinal ischemia/reperfusion in rats. BBRC 2009, 381, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Shimouchi, A.; Nose, K.; Shirai, M.; Kondo, T. Estimation of molecular hydrogen consumption in the human whole body after the ingestion of hydrogen-rich water. Adv. Exp. Med. Biol. 2012, 737, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Danicke, S.; Ueberschar, K.H.; Valenta, H.; Schnurrbusch, U.; Ganter, M.; Klobasa, F.; Flachowsky, G. Effects of graded levels of fusarium toxin contaminated maize in diets for female weaned piglets. Arch. Anim. Nutr. 2003, 57, 311–334. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Weng, C.F.; Chen, B.J.; Chang, M.H. Toxicity of different fusarium mycotoxins on growth performance, immune responses and efficacy of a mycotoxin degrading enzyme in pigs. Anim. Res. 2006, 55, 579–590. [Google Scholar] [CrossRef]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Guerra-Ordaz, A.A.; Molist, F.; Hermes, R.G.; Gómez de Segura, A.; La Ragione, R.M.; Woodward, M.J.; Tchorzewska, M.A.; Collins, J.W.; Pérez, J.F.; Martín-Orúe, S.M. Effect of inclusion of lactulose and lactobacillus plantarum on the intestinal environment and performance of piglets at weaning. Anim. Feed Sci. Technol. 2013, 185, 160–168. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kumari, S. Lactulose: Production, purification and potential applications. Biotechnol. Adv. 2011, 29, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Krueger, M.; Schroedl, W.; Isik, W.; Lange, W.; Hagemann, L. Effects of lactulose on the intestinal microflora of periparturient sows and their piglets. Eur. J. Nutr. 2002, 41 (Suppl. 1), 126–131. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.H.; Smedh, U.; Kinzig, K.P.; Scott, K.A.; Knipp, S.; Ladenheim, E.E. Peptide yy(3-36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R384–R388. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, H.R.; He, K.; Pan, X.; Sugita-Konishi, Y.; Watanabe, M.; Zhang, H.; Pestka, J.J. Role of cholecystokinin in anorexia induction following oral exposure to the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon x, and nivalenol. Toxicol. Sci. 2014, 138, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.M.; Clark, E.S.; Pestka, J.J. Anorexia induction by the trichothecene deoxynivalenol (vomitoxin) is mediated by the release of the gut satiety hormone peptide yy. Toxicol. Sci. 2012, 130, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.D.; Bates, M.A.; Bursian, S.J.; Flannery, B.; Zhou, H.R.; Link, J.E.; Zhang, H.B.; Pestka, J.J. Peptide yy3-36 and 5-hydroxytryptamine mediate emesis induction by trichothecene deoxynivalenol (vomitoxin). Toxicol. Sci. 2013, 133, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.M.; Irani, B.G.; Moore, M.C.; Haskell-Luevano, C.; Millard, W.J. Ghrelin-induced food intake and growth hormone secretion are altered in melanocortin 3 and 4 receptor knockout mice. Peptides 2005, 26, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Yamafuji, M.; Tachibana, T.; Nakabeppu, Y.; Noda, M.; Nakaya, H. Oral ‘hydrogen water’ induces neuroprotective ghrelin secretion in mice. Sci. Rep. 2013, 3, 3273. [Google Scholar] [CrossRef] [PubMed]
- Frankic, T.; Pajk, T.; Rezar, V.; Levart, A.; Salobir, J. The role of dietary nucleotides in reduction of DNA damage induced by t-2 toxin and deoxynivalenol in chicken leukocytes. Food Chem. Toxicol. 2006, 44, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Frankic, T.; Salobir, J.; Rezar, V. The effect of vitamin e supplementation on reduction of lymphocyte DNA damage induced by t-2 toxin and deoxynivalenol in weaned pigs. Anim. Feed Sci. Technol. 2008, 141, 274–286. [Google Scholar] [CrossRef]
- Xiao, H.; Wu, M.M.; Tan, B.E.; Yin, Y.L.; Li, T.J.; Xiao, D.F.; Li, L. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity. J. Anim. Sci. 2013, 91, 4772–4780. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Gao, J.; Liu, F.X.; Broomhead, J.; Chi, F. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J. Anim. Sci. 2011, 89, 3008–3015. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.F.; Zhang, N.Y.; Peng, Y.Z.; Qi, D.S. Interaction of zearalenone and soybean isoflavone in diets on the growth performance, organ development and serum parameters in prepubertal gilts. J. Anim. Physiol. Anim. Nutr. 2012, 96, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Zhang, J.H.; Cai, J.-M.; Cao, Y.-P.; Sun, X.-J. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced alzheimer’s disease by reduction of oxidative stress. Brain Res. 2010, 1328, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Liu, Q.; Yang, R.; Zhang, J.H.; Cao, Y.P.; Sun, X.J. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of jnk and nf-kappab activation in a rat model of amyloid-beta-induced alzheimer’s disease. Neurosci. Lett. 2011, 491, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Manyes, L.; Manes, J.; Meca, G. Influence of prebiotics, probiotics and protein ingredients on mycotoxin bioaccessibility. Food Funct. 2015, 6, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Florent, C.; Flourie, B.; Leblond, A.; Rautureau, M.; Bernier, J.J.; Rambaud, J.C. Influence of chronic lactulose ingestion on the colonic metabolism of lactulose in man (an in vivo study). J. Clin. Investig. 1985, 75, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Duan, Y.; Ge, C.; Chen, C.; Zhou, M. Functional analysis of the beta2 -tubulin gene of fusarium graminearum and the beta-tubulin gene of botrytis cinerea by homologous replacement. Pest Manag. Sci. 2013, 69, 582–588. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (U.S.). Committee on Nutrient Requirements of Swine. In Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012; pxvii; 4400p. [Google Scholar]
- Ji, F.; Xu, J.; Liu, X.; Yin, X.; Shi, J. Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu Province, China. Food Chem. 2014, 157, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Hou, Y.; Yao, W. Lactulose increases equol production and improves liver antioxidant status in barrows treated with daidzein. PLoS ONE 2014, 9, e93163. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, L.; Zhou, W.; Hu, L.; Li, L.; Tu, Q.; Chang, Y.; Liu, Q.; Sun, X.; Wu, M.; et al. The protective role of hydrogen-rich saline in experimental liver injury in mice. J. Hepatol. 2011, 54, 471–480. [Google Scholar] [CrossRef] [PubMed]

| Item | NC | MC | MC + LAC | MC + HRW | SEM | p-Value |
|---|---|---|---|---|---|---|
| BW, kg | ||||||
| Day 0 | 7.66 | 7.88 | 7.76 | 8.00 | 0.20 | 0.947 |
| Day 14 | 12.42 | 10.42 | 12.27 | 12.14 | 0.39 | 0.244 |
| Day 25 | 18.78 | 14.44 | 17.70 | 17.48 | 0.61 | 0.056 |
| ADG, kg/day | ||||||
| Days 0 to 14 | 0.34 a | 0.18 b | 0.32 a | 0.29 a | 0.02 | 0.013 |
| Days 14 to 25 | 0.58 | 0.36 | 0.49 | 0.48 | 0.03 | 0.055 |
| Days 0 to 25 | 0.44 a | 0.26 b | 0.40 a | 0.38 a | 0.02 | 0.008 |
| ADFI, g/day | ||||||
| Days 0 to 14 | 0.58 a | 0.39 b | 0.53 a | 0.58 a | 0.03 | 0.010 |
| Days 14 to 25 | 1.04 a | 0.66 b | 0.86 a,b | 0.87 a,b | 0.05 | 0.034 |
| Days 0 to 25 | 0.79 a | 0.51 b | 0.67 a | 0.71 a | 0.03 | 0.017 |
| G:F, g/g | ||||||
| Days 0 to 14 | 0.59 | 0.46 | 0.60 | 0.51 | 0.02 | 0.060 |
| Days 14 to 25 | 0.56 | 0.56 | 0.59 | 0.55 | 0.02 | 0.948 |
| Days 0 to 25 | 0.57 | 0.53 | 0.60 | 0.53 | 0.02 | 0.358 |
| Item | NC | MC | MC + LAC | MC + HRW | SEM | p-Value |
|---|---|---|---|---|---|---|
| Ghrelin (ng/L) | 3743.92 | 3064.05 | 3533.69 | 3587.87 | 85.79 | 0.112 |
| PYY (pg/mL) | 740.09 b | 837.44 a | 729.94 b | 727.60 b | 13.77 | 0.003 |
| CCK (ng/L) | 178.29 b | 211.72 a | 176.26 b | 157.63 b | 7.65 | 0.007 |
| Items | NC | MC | MC + LAC | MC + HRW | SEM | p-Value |
|---|---|---|---|---|---|---|
| Total carbonyl (mg/mL) | 0.78 b | 1.07 a | 0.84 b | 0.86 b | 0.04 | 0.047 |
| 8-OH-dG (ng/mL) | 8.41 b | 11.97 a | 8.24 b | 8.14 b | 0.48 | 0.002 |
| MDA (nmol/mL) | 4.15 a,b | 4.79 a | 3.23 b | 3.66 a,b | 0.29 | 0.043 |
| CAT (U/mL) | 2.54 | 3.96 | 2.52 | 2.86 | 0.23 | 0.079 |
| Total-SOD (U/mL) | 76.06 | 77.43 | 77.02 | 73.69 | 1.11 | 0.669 |
| CuZn-SOD (U/mL) | 72.20 | 70.94 | 74.16 | 69.33 | 1.20 | 0.576 |
| Mn-SOD (U/mL) | 6.06 | 6.48 | 5.86 | 6.56 | 0.19 | 0.562 |
| Items | NC | MC | MC + LAC | MC + HRW | SEM | p-Value |
|---|---|---|---|---|---|---|
| Total carbonyl (mg/g wt liver) | 1.64 a,b | 1.84 a | 1.58 b | 1.56 b | 0.04 | 0.028 |
| Inhibiting hydroxyl radical (U/mg protein) | 1979.50 b | 2444.81 a,b | 2777.68 b | 3140.79 a | 141.28 | 0.013 |
| Protein carbonyl (nmol/mg protein) | 5.63 | 5.26 | 5.91 | 6.52 | 0.25 | 0.369 |
| Lipid peroxidation (μmol/g protein) | 1.41 b | 2.09 a | 1.42 b | 1.41 b | 0.08 | <0.001 |
| MDA (nmol/mg protein) | 1.55 | 1.28 | 1.16 | 1.24 | 0.05 | 0.061 |
| CAT (U/mg protein) | 24.60 a,b | 27.95 a | 21.21 b,c | 19.19 c | 0.93 | <0.001 |
| Total-SOD (U/mg protein) | 206.61 a | 188.58 b | 212.62 a | 209.00 a | 3.20 | 0.023 |
| CuZn-SOD (U/mg protein) | 141.31 b,c | 137.12 c | 157.88 a | 153.58 a,b | 2.79 | 0.011 |
| Mn-SOD (U/mg protein) | 65.30 a | 49.45 b | 58.74 a,b | 57.42 a,b | 2.02 | 0.033 |
| GSH-px (U/mg protein) | 130.29 | 122.56 | 107.93 | 111.98 | 3.46 | 0.078 |
| T-GSH (μmol/g wt liver) | 1.84 | 1.80 | 1.80 | 2.09 | 0.11 | 0.801 |
| GSSG (μmol/g wt liver) | 0.59 | 0.51 | 0.66 | 0.57 | 0.03 | 0.199 |
| Reduced GSH (μmol/g wt liver) | 0.66 | 0.79 | 0.49 | 0.95 | 0.10 | 0.474 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Ji, X.; Zhang, Q.; Du, W.; Wei, Q.; Yao, W. Hydrogen-Rich Water and Lactulose Protect Against Growth Suppression and Oxidative Stress in Female Piglets Fed Fusarium Toxins Contaminated Diets. Toxins 2018, 10, 228. https://doi.org/10.3390/toxins10060228
Zheng W, Ji X, Zhang Q, Du W, Wei Q, Yao W. Hydrogen-Rich Water and Lactulose Protect Against Growth Suppression and Oxidative Stress in Female Piglets Fed Fusarium Toxins Contaminated Diets. Toxins. 2018; 10(6):228. https://doi.org/10.3390/toxins10060228
Chicago/Turabian StyleZheng, Weijiang, Xu Ji, Qing Zhang, Wenchao Du, Quanwei Wei, and Wen Yao. 2018. "Hydrogen-Rich Water and Lactulose Protect Against Growth Suppression and Oxidative Stress in Female Piglets Fed Fusarium Toxins Contaminated Diets" Toxins 10, no. 6: 228. https://doi.org/10.3390/toxins10060228
APA StyleZheng, W., Ji, X., Zhang, Q., Du, W., Wei, Q., & Yao, W. (2018). Hydrogen-Rich Water and Lactulose Protect Against Growth Suppression and Oxidative Stress in Female Piglets Fed Fusarium Toxins Contaminated Diets. Toxins, 10(6), 228. https://doi.org/10.3390/toxins10060228