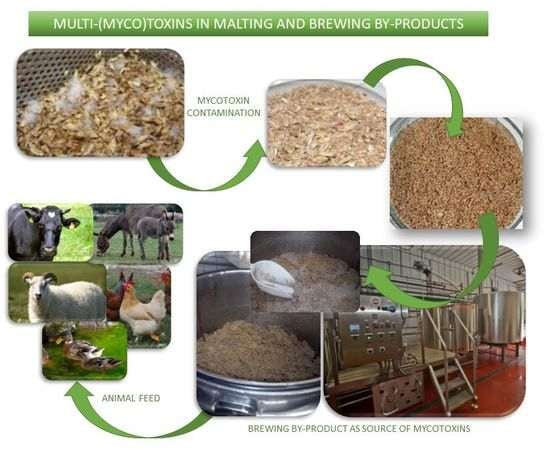
Multi-(myco)toxins in Malting and Brewing By-Products
Abstract
:1. Introduction
2. The Production of Malting and Brewing By-Products
3. Common Fungal Species in Malting and Brewing
4. General Toxicity and Occurrence of Multi- and Myco-Toxins
- Phase I includes: α-zearalenol, β-zearalenol (α-ZEL and β-ZEL), zearalenone-4-O-β-glucoside (ZEA-14Glc), zearalenone-16-O-β-glucoside (ZEA-16Glc), and zearalenone sulphate (ZEA-14-sulphate, ZEA-14S), and many of them have the ability to transform into their basic form during digestion in the mammalian intestinal tract [44]. ZEA-14Glc can generate complex compounds, such as zearalenone-malonyl-glucoside (ZEA-MalGlc), zearalenone-di-hexoside (ZEA-di-hexoside), and zearalenone-pentosylhexoside [45].
5. Multi- and Myco-Toxins in Malting and Brewing By-Products
6. Malting and Brewing By-Products Adding Value to Different Food Products
7. The Disposal/Use of Infected Grains
- Clean and blend—with the uncontaminated grain; exclusively for animal feed;
- Dumping—grains with high toxin levels can be disposed in a bush, slough, or in a hole;
- Gravity sorter—grains infected with Fusarium have a lower weight and are easy to sort with this technique;
- Burning—the most common method of disposal. This requires an investment in a grain-burning stove, but the produced heat can be used for heating. However, according to Agnew and Kirsch [86], ash can still contain DON and fungal spores and thus represents a danger to human health;
- Composting;
- Anaerobic digestion; and
- Gamma irradiation [88].
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mastanjević, K.; Šarkanj, B.; Warth, B.; Krska, R.; Sulyok, M.; Mastanjević, K.; Šantek, B.; Krstanović, V. Fusarium culmorum multi-toxin screening in malting and brewing byproducts. LWT 2018, 98, 642–645. [Google Scholar] [CrossRef]
- Mastanjević, K.; Šarkanj, B.; Šantek, B.; Mastanjević, K.; Krstanović, V. Fusarium culmorum mycotoxin transfer from wheat to malting and brewing products and by-products. World Mycotoxin J. 2018, in press. [Google Scholar] [CrossRef]
- MAGB. Available online: http://www.ukmalt.com/malting-co-products (accessed on 16 March 2018).
- Mussato, S.I. Biotechnological potential of brewing industry by-products. In Biotechnology for Agro-Industrial Residues Utilization; Singh nee’ Nigam, P., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 313–326. [Google Scholar]
- Krstanović, V.; Klapec, T.; Velić, N.; Milaković, Z. Contamination of malt barley and wheat by Fusarium graminearum and Fusarium culmorum from the crop years 2001–2003 in Eastern Croatia. Microbiol. Res. 2005, 160, 353–359. [Google Scholar] [CrossRef]
- Snijders, C.H.A. Systemic fungal growth of Fusarium culmorum in stems of winter wheat. J. Phytopathol. 1990, 129, 133–140. [Google Scholar] [CrossRef]
- Parikka, P.; Hakala, K.; Tiilikkala, K. Expected shifts in Fusarium species’ composition on cereal grain in Northern Europe due to climatic change. Food Addit. Contam. A 2012, 29, 1543–1555. [Google Scholar] [CrossRef]
- Ward, T.J.; Clear, R.M.; Rooney, A.P.; O’Donnell, K.; Gaba, D.; Patrick, S.; Starkey, D.E.; Gilbert, J.; Geiser, D.M.; Nowicki, T.W. An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genet. Biol. 2008, 45, 473–484. [Google Scholar] [CrossRef]
- Zhang, H.; Van der Lee, T.; Waalwijk, C.; Chen, W.; Xu, J.; Xu, J.; Zhang, Y.; Feng, L. Population analysis of the Fusarium graminearum species complex from wheat in china show a shift to more aggressive isolates. PLoS ONE 2012, 7, e31722. [Google Scholar] [CrossRef]
- Roháčik, T.; Hudec, K. Fungal infection of malt barley kernels in Slovak Republic. Plant Prot.Sci. 2007, 43, 86–93. [Google Scholar] [CrossRef]
- Gonzalez Pereyra, M.L.; Rosa, C.A.R.; Dalcero, A.M.; Cavaglieri, L.R. Mycobiota and mycotoxins in malted barley and brewer’s spent grain from Argentinean breweries. Lett. Appl. Microbiol. 2011, 53, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Piacentini, K.C.; Savi, G.D.; Pereira, M.E.V.; Scussel, V.M. Fungi and the natural occurrence of deoxynivalenol and fumonisins in malting barley (Hordeum vulgare L.). Food Chem. 2015, 187, 204–209. [Google Scholar] [CrossRef]
- Mastanjević, K.; Krstanović, V.; Mastanjević, K.; Šarkanj, B. Malting and Brewing Industries Encounter Fusarium spp. Related Problems. Fermentation 2018, 4, 3. [Google Scholar] [CrossRef]
- Habler, K.; Hofer, K.; Geissinger, C.; Schuler, J.; Huckelhoven, R.; Hess, M.; Gastl, M.; Rychlik, M. Fate of Fusarium toxins during the malting process. J. Agric. Food Chem. 2016, 64, 1377–1384. [Google Scholar] [CrossRef]
- CAST (Councile for Agricultural Science and Technology). Mycotoxins: Risk in Plant, Animal and Human Systems; Task Force Report No. 139; CAST: Ames, Iowa, 2003. [Google Scholar]
- Jarvis, B.B. Chemistry and toxicology of molds isolated from water-damaged buildings, Mycotoxins and Food Safety. Adv. Exp. Med. Biol. 2002, 504, 43–52. [Google Scholar]
- Pennsylvania Mycotoxin Management Guidance Document. Deoxynivalenol (DON) in Corn. Pennsylvania Department of Agriculture, Pennsylvania Department of Environmental Protection, 2012. Available online: https://www.agriculture.pa.gov/Food/pet_food/Documents/ManagementofDONincorn.pdf (accessed on 8 November 2018).
- Varga, E.; Wiesenberger, G.; Hametner, C.; Ward, T.J.; Dong, Y.; Schöfbeck, D.; McCormick, S.; Broz, K.; Stückler, R.; Schuhmacher, R.; et al. New tricks of an old enemy: Isolates of Fusarium graminearum produce a type A trichothecene mycotoxin. Environ. Microbiol. 2015, 17, 2588–2600. [Google Scholar] [CrossRef]
- Berthiller, F.; Sulyok, M.; Krska, R.; Schuhmacher, R. Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. Int. J. Food Microbiol. 2007, 119, 33–37. [Google Scholar] [CrossRef]
- Dupire, S. Mycotoxins and other contaminants in the malting and brewing industries. In Proceedings of the European Brewery Convention Congress, Dublin, Ireland, 17–22 May 2003. [Google Scholar]
- Melotte, L. Survey on the analysis of mycotoxins. Analysis Committee of the European Brewery Convention, European Brewery Association. J. Inst. Brew. 2004, 110, 235–239. [Google Scholar]
- Directive 2002/32/EC. OJEC, L140/10. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:aca28b8c-bf9d-444f-b470-268f71df28fb.0004.02/DOC_1&format=PDF (accessed on 8 January 2019).
- Recommendation 2006/576/EC. OJEC, L229/7. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:229:0007:0009:EN:PDF (accessed on 8 January 2019).
- Recommendation 2013/165/EC. OJEC L 91, 12–15. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:091:0012:0015:EN:PDF (accessed on 8 January 2019).
- Ruiz-Medina, A.; Fernández-de Córdova, M.L. Aflatoxin B1 in beer at different Stages of Production. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Elsevier/Academic Press: San Diego, CA, USA, 2015; pp. 517–523. [Google Scholar]
- Commission Regulation 2174/2003 EC. OJEC, L326, 12–15. Available online: https://publications.europa.eu/en/publication-detail/-/publication/c849b56b-42ec-4e34-8c90-3a3668737420 (accessed on 8 January 2019).
- World Health Organization International Agency for Research on Cancer IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 82. 2002. Available online: http://monographs.iarc.fr/ENG/Monographs/vol82/mono82.pdf (accessed on 8 July 2018).
- Mably, M.; Mankotia, M.; Cavlovic, P.; Tam, J.; Wong, L.; Pantazopoulos, P.; Calway, P.; Scott, P.M. Survey of aflatoxins in beer sold in Canada. Food Addit. Contam. 2005, 22, 1252–1257. [Google Scholar] [CrossRef]
- Nakajima, M.; Tsubouchi, H.; Miyabe, M. A survey of ochratoxin A and aflatoxins in domestic and imported beers in Japan by immunoaffinity and liquid chromatography. J. AOAC Int. 1999, 82, 897–902. [Google Scholar]
- Inoue, T.; Nagatomi, Y.; Uyama, A.; Mochizuki, N. Fate of mycotoxins during beer brewing and fermentation. Biosci. Biotechnol. Biochem. 2013, 77, 1410–1415. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Salleh, B. A preliminary study on the occurrence of Aspergillus spp. and aflatoxin B1 in imported wheat and barley in Penang, Malaysia. Mycotoxin Res. 2010, 26, 267–271. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, E.K.; Shon, D.H.; Kim, Y.B. Natural co-occurrence of aflatoxin B1, fumonisin B1 and ochratoxin A in barley and corn foods from Korea. Food Addit. Contam. 2002, 19, 1073–1080. [Google Scholar] [CrossRef]
- Scudamore, K.A.; Patel, S. Survey for aflatoxins, ochratoxin A, zearalenone and fumonisins in maize imported into United Kingdom. Food Addit. Contam. 2000, 17, 407–416. [Google Scholar] [CrossRef]
- Lewis, L.; Onsongo, M.; Njapau, H.; Shurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A.; et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767. [Google Scholar] [CrossRef]
- Gerbaldo, G.A.; Pereyra, C.M.; Cavaglieri, L.R.; Ruiz, F.; Pascual, L.; Dalcero, A.M.; Barberis, A.M. Surveillance of aflatoxin and microbiota related to brewers grain destined for swine feed in Argentina. Vet. Med. Int. 2011, 912480. [Google Scholar] [CrossRef]
- Papadopoulou-Bouraoui, A.; Vrabcheva, T.; Valzacchi, S.; Stroka, J.; Anklam, E. Screening survey of deoxynivalenol in beer from the European market by an enzyme-linked immunosorbent assay. Food Addit. Contam. 2004, 21, 607–617. [Google Scholar] [CrossRef]
- Milićević, D.R.; Škrinjar, M.; Baltić, T. Real and perceived risks for mycotoxin contamination in foods and feeds: Challenges for food safety control. Toxins 2010, 2, 527–592. [Google Scholar] [CrossRef]
- Salas, B.; Steffenson, B.J.; Casper, H.H.; Tacke, B.; Prom, L.K.; Fetch, T.G., Jr.; Schwarz, P.B. Fusarium species pathogenic to barley and their associated mycotoxins. Plant Dis. 1999, 83, 667–674. [Google Scholar] [CrossRef]
- Velić, N.; Pavlović, H.; Ćosić, J.; Kanižai, G.; Krstanović, V. A survey of Fusarium graminearum and deoxynivalenol contamination of malt barley from the crop year 2004 in eastern Croatia. Cereal Res. Commun. 2007, 35, 1293–1296. [Google Scholar] [CrossRef]
- Legzdina, l.; Buerstmayr, H. Comparison of infection with Fusarium head blight and accumulation of mycotoxins in grain of hulles and covered barley. J. Cereal Sci. 2004, 40, 61–67. [Google Scholar] [CrossRef]
- Rotter, B.A.; Prelusky, D.B. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar] [CrossRef]
- Saenz de Rodrigues, C.A.; Bongiovanni, A.M.; Conde de Borrego, L. An epidemic of precocious development in Puerto Rican children. J. Pediatr. 1985, 107, 393–396. [Google Scholar] [CrossRef]
- Campbell, G.D. Trichothecene mycotoxicosis B a new entity? S. Afr. Med. J. 1991, 80, 361–362. [Google Scholar]
- Dall’Erta, A.; Cirlini, M.; Dall’Asta, M.; Del Rio, D.; Galaverna, G.; Dall’Asta, C. Masked mycotoxins are efficiently hydrolyzed by human colonic microbiota releasing their aglycones. Chem. Res. Toxicol. 2013, 26, 305–312. [Google Scholar] [CrossRef]
- Berthiller, F.; Werner, U.; Sulyok, M.; Krska, R.; Hauser, M.-T.; Schuhmacher, R. Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) determination of phase II metabolites of the mycotoxin zearalenone in the model plant Arabidopsis thaliana. Food Addit. Contam. 2006, 23, 1194–1200. [Google Scholar] [CrossRef]
- González Pereyra, M.L.; Sulyok, M.; Baralla, V.; Dalcero, A.M.; Krska, R.; Chulze, S.; Cavaglieri, L.R. Evaluation of zearalenone, -zearalenol, -zearalenol, zearalenone 4-sulfate and -zearalenol 4-glucoside levels during the ensiling process. World Mycotoxin J. 2014, 7, 291–295. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef]
- Binder, S.B.; Schwartz-Zimmermann, H.E.; Varga, E.; Bichl, G.; Michlmayr, H.; Adam, G.; Berthiller, F. Metabolism of zearalenone and its major modified forms in pigs. Toxins 2017, 9, 56. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Battilani, P. Fumonisins and their modified forms, a matter of concern in future scenario? World Mycotoxin J. 2016, 9, 727–739. [Google Scholar] [CrossRef]
- Scott, P.M.; Lawrence, G.A. Analysis of beer for fumonisins. J. Food Prot. 1995, 58, 1379–1382. [Google Scholar] [CrossRef]
- Daško, Ľ.; Rauová, D.; Belajová, E.; Kováč, M. Determination of fumonisins B1 and B2 in beer. Czech. J. Food Sci. 2005, 23, 20–26. [Google Scholar] [CrossRef]
- Voss, K.A.; Riley, R.T. Fumonisin toxicity and mechanism of action: Overview and current perspectives. Food Saf. 2013, 1, 49–69. [Google Scholar] [CrossRef]
- Scott, P.M. Recent research on fumonisins: A review. Food Addit. Contam. A 2012, 29, 242–248. [Google Scholar] [CrossRef]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill, A.H., Jr.; Rothman, K.J.; Hendricks, K.A. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ. Health Perspect. 2006, 114, 237–241. [Google Scholar] [CrossRef]
- Rheeder, J.; Marasas, W.; Theil, P.; Sydenham, E.; Shephard, G.; Van Schalkwyk, D. Fusarium moniliforme and fumonisins in corn in relation to human esophageal cancer in Transkei. Phytopathology 1992, 82, 353–357. [Google Scholar] [CrossRef]
- Braun, M.S.; Wink, M. Exposure, occurrence, and chemistry of fumonisins and their cryptic derivatives. Compr. Rev. Food Sci. Food Saf. 2018, 17, 769–791. [Google Scholar] [CrossRef]
- Maranghi, F.; Tassinari, R.; Narciso, L.; Tait, S.; Rocca, C.L.; Felice, G.D.; Butteroni, C.; Corinti, S.; Barletta, B.; Cordelli, E.; et al. In Vivo Toxicity and Genotoxicity of Beauvericin and Enniatins. Combined Approach to Study In Vivo Toxicity and Genotoxicity of Mycotoxins Beauvericin (BEA) and Enniatin B (ENNB). EFSA Supporting Publication 2018, 15, 1406E. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2018.EN-1406 (accessed on 8 January 2019). [CrossRef]
- Anli, E.; Mert Alkis, I. Ochratoxin A and brewing technology: A Review. J. Inst. Brew. 2010, 116, 23–32. [Google Scholar] [CrossRef]
- Creppy, E.E. Human ochratoxicosis. J. Toxicol. 1999, 18, 277–293. [Google Scholar] [CrossRef]
- Huff, W.E.; Doerr, J.A. Synergism between aflatoxin and ochratoxin A in broiler chickens. Poult. Sci. 1981, 60, 550–555. [Google Scholar] [CrossRef]
- Peraica, M.; Rašić, D. The impact of mycotoxicoses on human history. Arch. Ind. Hyg. Toxicol. 2012, 63, 513–518. [Google Scholar] [CrossRef]
- Veprikova, Z.; Vaclavikova, M.; Lacina, O.; Dzuman, Z.; Zachariasova, M.; Hajslova, J. Occurrence of mono and di-glycosylated conjugates of T-2 and HT-2 toxins in naturally contaminated cereals. World Mycotoxin J. 2012, 5, 231–240. [Google Scholar] [CrossRef]
- Nakagawa, H.; Sakamoto, S.; Sago, Y.; Nagashima, H. Detection of type A trichothecene di-glucosides produced in corn by high-resolution liquid chromatography-orbitrap mass spectrometry. Toxins 2013, 5, 590–604. [Google Scholar] [CrossRef]
- McCormick, S.P.; Price, N.P.J.; Kurtzman, C.P. Glucosylation and other biotransformations of T-2 toxin by yeasts of the Trichomonascus clade. Appl. Environ. Microbiol. 2012, 78, 8694–8702. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Varga, E.; Meng-Reiterer, J.; Bueschl, C.; Michlmayr, H.; Malachova, A.; Fruhmann, P.; Jestoi, M.; Peltonen, K.; Adam, G.; et al. Metabolism of the Fusarium mycotoxins T-2 toxin and HT-2 toxin in wheat. J. Agric. Food Chem. 2015, 63, 7862–7872. [Google Scholar] [CrossRef]
- Wen-Shyg Chiou, P.; Chen, C.R.; Chen, K.J.; Yu, B. Wet brewers’ grains or bean curd pomance as partial replacement of soybean meal for lactating cows. Anim. Feed Sci. Technol. 1998, 74, 123–134. [Google Scholar] [CrossRef]
- Cavaglieri, L.R.; Keller, K.M.; Pereya, C.M.; Gonzalez Pereya, M.L.; Alonso, V.A.; Rojo, F.G.; Dalcero, A.M.; Rosa, C.A.R. Fungi and natural incidence of selected mycotoxins in barley rootlets. J. Stored Prod. Res. 2009, 45, 147–150. [Google Scholar] [CrossRef]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011, 120, S28–S48. [Google Scholar] [CrossRef]
- Habschied, K.; Šarkanj, B.; Klapec, T.; Krstanović, V. Distribution of zearalenone in malted barley fractions dependent on Fusarium graminearum growing conditions. Food Chem. 2011, 129, 329–332. [Google Scholar] [CrossRef]
- Habschied, K.; Šarkanj, B.; Krstanović, V.; Velić, N.; Novak, M.; Mastanjević, K. Fusarium mycotoxins in malting and brewing by-products. In Proceedings of the World Mycotoxin Forum 8th Conference, Mycotoxin Control: The System Approach, Vienna, Austria, 10–12 November 2014; p. 85. [Google Scholar]
- Krstanović, V.; Mastanjević, K.; Velić, N.; Pleadin, J.; Perši, N.; Španić, V. The influence of Fusarium culmorum contamination level on deoxynivalenol content in wheat, malt and beer. Roman. Biotechnol. Lett. 2015, 20, 10901–10910. [Google Scholar]
- Blezinger, S.B. Feed Supplements Come in Several Different Forms: Part 4. Cattle Today Online. Available online: http://www.cattletoday.com/archive/2003/February/CT251.shtml (accessed on 16 March 2018).
- Ha, J.-K.; Kim, S.W.; Kim, W.Y. Use of Agro-Industrial by-Products as Animal Feeds in Korea; ASPAC Food & Fertilizer Technology Center: Taipei, Taiwan, 1996; pp. 1–15. [Google Scholar]
- Rychlik, M.; Humpf, H.U.; Marko, D.; Dänicke, S.; Mally, A.; Berthiller, F.; Klaffke, H.; Lorenz, N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res. 2014, 30, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Maul, R.; Pielhau, R.; Koch, M. Evaluation of an extraction method and spin column cleanup procedure for Fusarium mycotoxins and their masked derivatives from grain matrix. Food Control 2014, 40, 151–156. [Google Scholar] [CrossRef]
- De Boevre, M.; Di Mavungu, J.D.; Landschoot, S.; Audenaert, K.; Eeckhout, M.; Maene, P.; Haesaert, G.; De Saeger, S. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin J. 2012, 5, 207–219. [Google Scholar] [CrossRef]
- Zachariasova, M.; Dzuman, Z.; Veprikova, Z.; Hajkova, K.; Jiru, M.; Vaclavikova, M.; Pospichalova, M.; Florian, M.; Hajslova, J. Occurrence of multiple mycotoxins in European feedingstuffs, assessment of dietary intake by farm animals. Anim. Feed Sci. Technol. 2014, 193, 124–140. [Google Scholar] [CrossRef]
- Jozinović, A.; Šubarić, D.; Ačkar, Đ.; Miličević, B.; Babić, J.; Jašić, M.; Lendić, V.K. Food industry by-products as raw materials in functional food production. Hrana u Zdravlju i Bolesti 2014, 3, 22–30. [Google Scholar]
- Obradović, V.; Babić, J.; Šubarić, D.; Ačkar, Đ.; Jozinović, A. Improvement of nutritional and functional properties of extruded food products. J. Food Nutr. Res. 2014, 53, 189–206. [Google Scholar]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Moreira, M.M.; Morais, S.; Carvalho, D.O.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. Brewer’s spent grain from different types of malt: Evaluation of the antioxidant activity and identification of the major phenolic compounds. Food Res. Int. 2013, 54, 382–388. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, C.; Connolly, A.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Phenolic extracts of brewers’ spent grain (BSG) as functional ingredients-Assessment of their DNA protective effect against oxidant-induced DNA single strand breaks in U937 cells. Food Chem. 2012, 134, 641–646. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Chaurin, V.; Reis, S.; Gallagher, E. Brewer’s spent grain as a functional ingredient for breadsticks. Int. J. Food Sci. Technol. 2012, 47, 1765–1771. [Google Scholar] [CrossRef] [Green Version]
- Corwell, C. Spent Grain Regulation: Commentary from the Beer Institute. 2014. Available online: https://www.craftbrewingbusiness.com/business-marketing/spent-grain-regulation-commentary-beer-institute/ (accessed on 16 March 2018).
- Scott, T. Feed and Processing Options for Heavily Downgraded Wheat. 2014. Available online: https://cigi.ca/wp-content/uploads/2014/11/Tom-Scott-Feed-and-Processing-Options-for-Heavily-Downgraded-Wheat.pdf (accessed on 12 December 2017).
- Agnew, J.; Kirsch, J. Evaluation of Disposal Options for Fusarium Damaged Grain and Screenings. 2017. Available online: http://pami.ca/wp-content/uploads/2017/04/R8415-Research-Report.pdf (accessed on 16 March 2018).
- King, C. Extracting Value from Fusariem-Damage Grain and Screenings. 2017. Available online: https://www.topcropmanager.com/diseases/from-waste-to-worth-20793 (accessed on 4 August 2018).
- Snizhko, O.; Lomova, N.; Narizhnyy, S.; Mingaleeva, Z. Enhancing food safety of pollen by means of irradiation. Ukr. Food J. 2015, 4, 32–39. Available online: http://ufj.ho.ua/Archiv/UKRAINIAN%20FOOD%20JOURNAL%202015%20V.4%20Is.1.pdf (accessed on 15 November 2018).
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glycoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Fruhmann, P.; Wiesenberger, G.; Kluger, B.; Sarkanj, B.; Lemmens, M.; Hametner, C.; Fröhlich, J.; Adam, G.; Krska, R.; et al. Deoxynivalenol-sulfates: Identification and quantification of novel conjugated (masked) mycotoxins in wheat. Anal. Bioanal. Chem. 2015, 407, 1033–1039. [Google Scholar] [CrossRef]
- Mastanjević, K.; Španić, V.; Horvat, D.; Mastanjević, K.; Šarkanj, B.; Krstanović, V.; Šantek, B. Establishing the impact of Fusarium culmorum infection and fungicide treatment on wheat malt quality. J. Food Process. Preserv. 2018, 42, e13714. [Google Scholar] [CrossRef]




| Mycotoxins | Source | ||
|---|---|---|---|
| Fungal | Fusarium mycotoxins | Deoxynivalenol, Nivalenol, 3-Acetyldeoxynivalenol, 15-Acetyldeoxynivalenol, Zearalenon, HT-2 toxin, T2-toxin, Moniliformin, Chrysogin, Culmorin,, Aurofusarin, Fumonisin B1, Fumonisin B2, Fumonisin B3, Beauvericin, Enniantins (A, A1, B, B1) | [1,2,12,15,77] |
| Other species mycotoxins (Aspergillus, Alternaria) | Aflatoxin B1, Tentoxin, Brevianamid F | [1,11,77] | |
| Yeast metabolite | Tryptophol | [1] | |
| Plant metabolites | DON-3-glucoside, Lotaustralin, Linamarin | [1,77] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastanjević, K.; Lukinac, J.; Jukić, M.; Šarkanj, B.; Krstanović, V.; Mastanjević, K. Multi-(myco)toxins in Malting and Brewing By-Products. Toxins 2019, 11, 30. https://doi.org/10.3390/toxins11010030
Mastanjević K, Lukinac J, Jukić M, Šarkanj B, Krstanović V, Mastanjević K. Multi-(myco)toxins in Malting and Brewing By-Products. Toxins. 2019; 11(1):30. https://doi.org/10.3390/toxins11010030
Chicago/Turabian StyleMastanjević, Kristina, Jasmina Lukinac, Marko Jukić, Bojan Šarkanj, Vinko Krstanović, and Krešimir Mastanjević. 2019. "Multi-(myco)toxins in Malting and Brewing By-Products" Toxins 11, no. 1: 30. https://doi.org/10.3390/toxins11010030
APA StyleMastanjević, K., Lukinac, J., Jukić, M., Šarkanj, B., Krstanović, V., & Mastanjević, K. (2019). Multi-(myco)toxins in Malting and Brewing By-Products. Toxins, 11(1), 30. https://doi.org/10.3390/toxins11010030