LC–MS/MS Analysis of the Emerging Toxin Pinnatoxin-G and High Levels of Esterified OA Group Toxins in Galician Commercial Mussels
Abstract
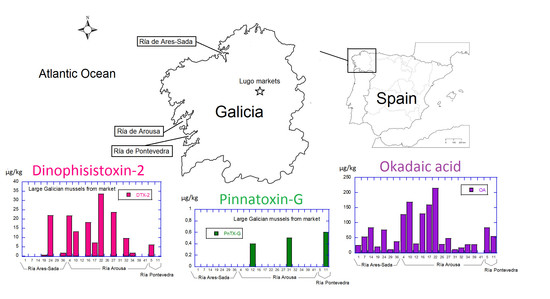
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Market Mussel Collection and Sample Preparation
4.2. Chemicals
4.3. Toxin Extraction and Hydrolysis Procedure
4.4. LC–MS Analysis
4.5. Method Performance, Recovery, and Matrix Correction
4.6. Expression of Results
Author Contributions
Funding
Conflicts of Interest
References
- Hallegraeff, G. Harmful algae and their toxins: Progress, paradoxes and paradim shifts, Toxins and Biologically Active Compounds from Microalgae. In Origin, Chemistry and Detection; Rossini, G., Ed.; CRC Press: Boca Raton, FL, USA, 2014; Volume 1, pp. 3–20. [Google Scholar]
- Ministry of Agriculture, Fisheries and Food (Government of Spain). Engorde Galicia Mejillón (Mytilus galloprovincialis). 2018. Available online: https://www.mapa.gob.es/app/jacumar/datos_produccion/lista_datos_produccion.aspx?Id=es (accessed on 28 April 2019).
- European Commission. Regulation (EU) No 15/2011 of 10 January of 2011 amending Regulation (EC) No 2074/2005 as regards recognised testing methods for detecting marine biotoxins in live bivalve molluscs. Off. J. Eur. Union 2011, L6, 3–6. [Google Scholar]
- European Union Reference Laboratory for Marine Biotoxins. EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Molluscs by LC-MS/MS. Version 5. 2015. Available online: http://www.aecosan.msssi.gob.es/AECOSAN/docs/documentos/laboratorios/LNRBM/ARCHIVO2EU-Harmonised-SOP-LIPO-LCMSMS_Version5.pdf (accessed on 28 April 2019).
- Codex Alimentarius International Food Standards. Standard for Live And raw Bivalve Molluscs Codex Standard 292-2008. Adopted in 2008. Amendment: 2013. Revision: 2014 and 2015. Food and Agriculture Organization of the United Nations; World Helth Organization, 2008. Available online: http://bach.cirsfid.unibo.it/node/portalfao/akn/fao/doc/standard/2008/CODEXSTAN292-2008/eng@2013/main.pdf (accessed on 28 April 2019).
- James, K.; Bishop, A.; Furey, A. New Toxins on The Horizon. In Seafood and Freshwater Toxins. Pharmacology, Physiology and Detection (Food Science and Technology), 1st ed.; Botana, L.M., Ed.; CRC Press Hardcover: New York, NY, USA, 2000; pp. 821–834. [Google Scholar]
- Munday, R. Imines: Gymnodimine, Spirolides, Pinnatoxins, Pteriatoxins, Prorocentrolide, Spiro-Prorocentrimine, and Symbioimines. In Seafood and Freshwater Toxins. Pharmacology, Physiology and Detection, 2nd ed.; Botana, L.M., Ed.; CRC Press Taylor and Francis Group: New York, NY, USA, 2008; pp. 581–594. [Google Scholar]
- Otero, P.; Alfonso, A.; Alfonso, C.; Aráoz, R.; Molgó, J.; Vieytes, M.; Botana, L. First direct fluorescence polarization assay for the detection and quantification of spirolides in mussel samples. Anal. Chim. Acta 2011, 701, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Molgó, J.; Marchot, P.; Aráoz, R.; Benoit, E.; Iorga, B.; Zakarian, A.; Taylor, P.; Bourne, Y.; Servent, D. Cyclic imine toxins from dinoflagellates: A growing family of potent antagonists of the nicotinic acetylcholine receptors. J. Neurochem. 2017, 142 (Suppl. 2), 41–51. [Google Scholar]
- Otero, P.; Alfonso, A.; Rodríguez, P.; Rubiolo, J.; Cifuentes, J.; Bermúdez, R.; Vieytes, M.; Botana, L. Pharmacokinetic and toxicological data of spirolides after oral and intraperitoneal administration. Food Chem. Toxicol. 2012, 50, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Curtis, J.; Oshima, Y.; Quilliam, M.; Walter, J.A.; WatsonWright, W.; Wright, J. Spirolides B and D, two novel macrocycles isolated from the disgestive glands of shellfish. J. Chem. Soc. Chem. Commun. 1995, 20, 2159–2161. [Google Scholar] [CrossRef]
- Guéret, S.; Brimble, M. Spiroimine shellfish poisoning (SSP) and the spirolide family of shellfish toxins: Isolation, structure, biological activity and synthesis. Nat. Prod. Rep. 2010, 27, 1350–1366. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Alfonso, A.; Alfonso, C.; Vieytes, M.R.; Louzao, M.C.; Botana, A.M.; Botana, L.M. New protocol to obtain spirolides from Alexandrium ostenfeldii cultures with high recovery and purity. Biomed. Chromatogr. 2010, 24, 878–886. [Google Scholar]
- Aasen, J.; MacKinnon, S.; LeBlanc, P.; Walter, J.A.; Hovgaard, P.; Aune, T.; Quilliam, M. Detection and identificationof spirolides in norwegian shellfish and plankton. Chem. Res. Toxicol. 2005, 18, 509–515. [Google Scholar] [CrossRef]
- Villar-González, A.; Rodríguez-Velasco, M.L.; Ben-Gigirey, B.; Botana, L.M. Lipophilic toxin profile in Galicia (Spain): 2005 toxic episode. Toxicon 2007, 49, 1129–1134. [Google Scholar] [CrossRef]
- Villar González, A.; Rodríguez-Velasco, M.; Ben-Gigirey, B.; Botana, L. First evidence of spirolides in Spanish shellfish. Toxicon 2006, 48, 1068–1074. [Google Scholar] [CrossRef]
- García-Altares, M.; Casanova, A.; Bane, V.; Diogène, J.; Furey, A.; de la Iglesia, P. Confirmation of pinnatoxins and spirolides in shellfish and passive samplers from Catalonia (Spain) by liquid chromatography coupled with triple quadrupole and high-resolution hybrid tandem mass spectrometry. Mar. Drugs 2014, 12, 3706–3732. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; González, V.; Martínez, A.; Paz, B.; Lago, J.; Cordeiro, V.; Blanco, L.; Vieites, J.; Cabado, A. Occurrence of Lipophilic Marine Toxins in Shelfish from Galica (NW of Spain) and Synergies among them. Mar. Drugs 2015, 13, 1666–1687. [Google Scholar] [CrossRef] [PubMed]
- Rundberget, T.; Aasen, J.; Selwood, A.; Miles, C. Pinnatoxins and spirolides in Norwegian blue mussels and seawater. Toxicon 2011, 58, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Rambla-Alegre, M.; Miles, C.; de la Iglesia, P.; Fernandez-Tejedor, M.; Jacobs, S.; Sioen, I.; Verbeke, W.; Samdal, I.; Sandvik, M.; Barbosa, V.; et al. Occurrence of cyclic imines in European commercial seafood and consumers risk assessment. Environ. Res. 2018, 161, 392–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Regulation (EC) No 853/2004 of the European Parliament and of the Council ofApril 29, 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur. Commun. 2004, L139, 55–205. [Google Scholar]
- Rodríguez, I.; Alfonso, A.; Antelo, A.; Alvarez, M.; Botana, L. Evaluation of the Impact of Mild Steaming and Heat Treatment on the Concentration of Okadaic Acid, Dinophysistoxin-2 and Dinophysistoxin-3 in Mussels. Toxins 2016, 8, 175. [Google Scholar] [CrossRef]
- Fernández, M.L.; Reguera, B.; González-Gil, S.; Míguez, A. Pectenotoxin-2 in single-cell isolates of Dinophysis caudata and Dinophysis acuta from the Galician Rias (NW Spain). Toxicon 2006, 48, 477–490. [Google Scholar] [CrossRef]
- Blanco, J.; Arévalo, F.; Moroño, A.; Correo, J.; Muñíz, S.; Mariño, C.; Martín, H. Presence of azaspiracids in bivalve molluscs from Northern Spain. Toxicon 2017, 137, 135–143. [Google Scholar] [CrossRef]
- Otero, P.; Alfonso, A.; Vieytes, M.R.; Cabado, A.G.; Vieites, J.M.; Botana, L.M. Effects of environmental regimens on the toxin profile of Alexandrium ostenfeldii. Environ. Toxicol. Chem. 2010, 29, 301–310. [Google Scholar] [CrossRef]
- Salgado, P.; Riobó, P.; Rodríguez, F.; Franco, J.; Bravo, I. Differences in the toxin profiles of Alexandrium ostenfeldii (Dinophyceae) strains isolated from different geographic origins: Evidence of paralytic toxin, spirolide, and gymnodimine. Toxicon 2015, 103, 85–98. [Google Scholar] [CrossRef]
- Hess, P.; Abadie, E.; Hervé, F.; Berteaux, T.; Séchet, V.; Aráoz, R.; Molgó, J.; Zakarian, A.; Sibat, M.; Rundberget, T.; et al. Pinnatoxin G is responsible for atypical toxicity in mussels (Mytilus galloprovincialis) and clams (Venerupis decussata) from Ingril, a French Mediterranean lagoon. Toxicon 2013, 75, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Rehmann, N.; Philipp Hess, P.; Michael, A.; Quilliam, M. Discovery of new analogs of the marine biotoxin azaspiracid in blue mussels (Mytilus edulis) by ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Krocka, B.; Tillmanna, U.; Tebbena, J.; Trefaultb, N.; Haifeng Guc, H. Two novel azaspiracids from Azadinium poporum, and a comprehensive compilation of azaspiracids produced by Amphidomataceae, (Dinophyceae). Harmful Algae 2019, 82, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Torgersen, T.; Sandvik, M.; Lundve, B.; Lindegarth, S. Profiles and levels of fatty acid esters of okadaic acid group toxins and pectenotoxins during toxin depuration. Part II: Blue mussels (Mytilus edulis) and flat oyster (Ostrea edulis). Toxicon 2008, 52, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ota, H.; Yamasaki, M. Direct evidence of transformation of dinophysistoxin-1 to 7-O-acyldinophysistoxin-1 (dinophysistoxin-3) in the scallop Patinopecten yessoensis. Toxicon 1999, 37, 187–198. [Google Scholar] [CrossRef]
- Blanco, J.; Álvarez, G.; Rengel, J.; Díaz, R.; Mariño, C.; Martín, H.; Uribe, E. Accumulation and Biotransformation of Dinophysis Toxins by the Surf Clam Mesodesma donacium. Toxins 2018, 10, 314. [Google Scholar] [CrossRef] [PubMed]
- Dhanji-Rapkova, M.; O’Neill, A.; Maskrey, B.; Coates, L.; Teixeira Alves, M.; Kelly, R.; Hatfield, R.; Rowland-Pilgrim, S.; Lewis, A.; Algoet, M.; et al. Variability and profiles of lipophilic toxins in bivalves from Great Britain during five and a half years of monitoring: Okadaic acid, dinophysistoxins and pectenotoxins. Harmful Algae 2018, 77, 66–80. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission decision 2002/657EC implementing Council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, L221, 8–36. [Google Scholar]
- EFSA Panel. Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on Marine Biotoxins in Shellfish—Summary on regulated marine biotoxins. EFSA J. 2009, 1306, 1–23. [Google Scholar]




| Toxin | Curves | R2 | Recovery (%) | SSE (%) |
|---|---|---|---|---|
| Free OA | y = 2381.975x − 561.583 | 0.9977 | 76.03 ± 15.28 | 211.70 ± 19.54 |
| Free DTX-2 | y = 2013.034x + 633.298 | 0.9937 | 85.24 ± 4.29 | 181.51 ± 6.85 |
| PTX-2 | y = 34362.389x − 5660.310 | 0.9987 | 92.34 ± 4.88 | 88.27 ± 6.37 |
| AZA-2 | y = 543036.015x + 83486.877 | 0.9962 | 98.8 ± 4.80 | 33.14 ± 1.65 |
| SPX-13 | y = 581714.049x + 292701.316 | 0.9997 | 110.48 ± 0.86 | 70.27 ± 6.75 |
| PnTX-G | y = 387054.739x + 39641.076 | 0.9994 | 90.95 ± 1.61 | 64.74 ± 3.58 |
| Hydrolyzed OA | y = 1956.583x − 217.441 | 0.9992 | 63. 28 ± 1.61 | 162.37 ± 14.85 |
| Hydrolyzed DTX-2 | y = 1702.876x + 585.842 | 0.9991 | 62.63 ± 1.63 | 160.25 ± 3.81 |
| Sample No. | Free OA (µg/kg) | Total OA (µg/kg) | Free DTX-2 (µg/kg) | Total DTX-2 (µg/kg) | μg OA eq/kg | PTX-2 (µg/kg) | μg PTX eq/kg | AZA-2 (µg/kg) | μg AZA eq/kg | SPX-13 (µg/kg) | PnTX-G (µg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.4 | 23.9 | ~ | ~ | 23.9 | ~ | ~ | ~ | ~ | ~ | |
| 2 | ~ | 6.6 | ~ | ~ | 6.6 | 1.7 | 1.7 | ~ | ~ | ~ | |
| 3 | ~ | ~ | ~ | ~ | ~ | ~ | ~ | ~ | ~ | ~ | |
| 4 | ~ | 127.2 | ~ | 21.7 | 140.2 | ~ | ~ | ~ | ~ | ~ | |
| 5 | 8.8 | 83.0 | 0.4 | 6.4 | 86.8 | 1.8 | 1.8 | 1.8 | 3.2 | 12.7 | ~ |
| 6 | 6.5 | 105.0 | ~ | 3.3 | 107.0 | ~ | ~ | ~ | ~ | ~ | |
| 7 | ~ | 52.8 | ~ | ~ | 52.8 | ~ | ~ | ~ | ~ | ~ | |
| 8 | ~ | 36.9 | ~ | 5.5 | 40.2 | ~ | ~ | ~ | ~ | ~ | |
| 9 | 13.1 | 128.6 | 3.3 | 17.4 | 139.0 | 2.3 | 2.3 | 1.1 | 2.0 | 16.2 | 0.9 |
| 10 | ~ | 167.5 | ~ | 13.2 | 175.4 | ~ | ~ | ~ | ~ | ~ | |
| 11 | 4.4 | 54.0 | ~ | ~ | 54.0 | 1.8 | 1.8 | ~ | 5.4 | 0.6 | |
| 12 | 1.8 | 30.1 | ~ | ~ | 30.1 | 1.0 | 1.0 | ~ | 4.4 | 0.4 | |
| 13 | 6.0 | 86.3 | ~ | ~ | 86.3 | 1.2 | 1.2 | ~ | 11.6 | ~ | |
| 14 | 7.3 | 83.2 | ~ | ~ | 83.2 | 2.4 | 2.4 | ~ | 4.4 | ~ | |
| 15 | 4.3 | 27.2 | 0.3 | 2.7 | 8.8 | 1.7 | 1.7 | ~ | 4.0 | 0.4 | |
| 16 | 8.5 | 129.6 | 6.3 | 18.2 | 140.5 | 1.3 | 1.3 | ~ | 7.4 | ~ | |
| 17 | ~ | 158.9 | ~ | 7.0 | 163.1 | 0.7 | 0.7 | 0.9 | 1.6 | 13.4 | ~ |
| 18 | 12.2 | 56.0 | 0.5 | 0.9 | 56.5 | 1.4 | 1.4 | 0.9 | 1.6 | 28.9 | ~ |
| 19 | 1.7 | 18.9 | ~ | 0.7 | 19.3 | 0.8 | 0.8 | ~ | 2.6 | ~ | |
| 20 | 2.3 | 19.1 | 0.8 | 5.5 | 22.4 | 1.4 | 1.4 | ~ | 3.5 | ~ | |
| 21 | 13.9 | 113.0 | 3.6 | 16.1 | 122.6 | 2.9 | 2.9 | ~ | 13.3 | ~ | |
| 22 | 30.2 | 214.0 | 11.0 | 33.5 | 234.1 | ~ | ~ | ~ | ~ | ~ | |
| 23 | 7.1 | 55.6 | 1.1 | 5.2 | 58.7 | 1.9 | 1.9 | 0.9 | 1.6 | 14.7 | ~ |
| 24 | 7.5 | 75.3 | 3.1 | 21.9 | 88.4 | ~ | ~ | ~ | ~ | ~ | |
| 25 | ~ | 3.6 | ~ | ~ | 3.6 | ~ | ~ | ~ | 0.6 | ~ | |
| 26 | ~ | 28.3 | ~ | ~ | 28.3 | ~ | ~ | ~ | ~ | ~ | |
| 27 | 2.3 | 48.1 | 4.7 | 23.5 | 62.2 | ~ | ~ | 1.5 | 2.7 | 9.9 | ~ |
| 28 | ~ | 19.7 | ~ | ~ | 19.7 | ~ | ~ | ~ | 11.9 | ~ | |
| 29 | ~ | 9.8 | ~ | ~ | 9.8 | ~ | ~ | ~ | ~ | ~ | |
| 30 | ~ | 7.7 | ~ | ~ | 7.7 | ~ | ~ | ~ | ~ | ~ | |
| 31 | ~ | 10.7 | ~ | ~ | 10.7 | ~ | ~ | ~ | ~ | 0.5 | |
| 32 | ~ | 17.0 | ~ | 9.6 | 22.76 | ~ | ~ | ~ | ~ | ~ | |
| 33 | ~ | 22.0 | ~ | ~ | 22.0 | ~ | ~ | ~ | 16.3 | ~ | |
| 34 | 0.9 | 27.3 | ~ | 1.7 | 28.3 | ~ | ~ | ~ | ~ | ~ | |
| 35 | ~ | 23.2 | ~ | ~ | 23.2 | ~ | ~ | ~ | 10.8 | ~ | |
| 36 | ~ | 37.8 | ~ | 1.6 | 38.8 | ~ | ~ | ~ | ~ | ~ | |
| 37 | ~ | 7.8 | ~ | 1.9 | 8.9 | ~ | ~ | ~ | ~ | ~ | |
| 38 | ~ | 27.0 | ~ | ~ | 27.0 | ~ | ~ | ~ | ~ | ~ | |
| 39 | ~ | 27.1 | ~ | ~ | 27.1 | ~ | ~ | ~ | ~ | ~ | |
| 40 | ~ | ~ | ~ | ~ | ~ | ~ | ~ | ~ | ~ | ~ | |
| 41 | ~ | ~ | ~ | ~ | ~ | ~ | ~ | ~ | ~ | ~ |
| Sample | Year | Collection and Purchase | Commercial | Mussel |
|---|---|---|---|---|
| No. | Week No | Brand | Size | |
| 1 | 49 | A | Large | |
| 2 | 49 | B | Small | |
| 3 | 49 | C | Small | |
| 4 | 49 | D | Large | |
| 5 | 49 | E | Large | |
| 6 | 49 | E | Small | |
| 7 | 50 | A | Large | |
| 8 | 50 | B | Small | |
| 9 | 50 | C | Small | |
| 10 | 2018 | 50 | D | Large |
| 11 | 50 | E | Large | |
| 12 | 50 | B | Large | |
| 13 | 50 | C | Small | |
| 14 | 51 | A | Large | |
| 15 | 51 | B | Small | |
| 16 | 51 | C | Large | |
| 17 | 51 | D | Large | |
| 18 | 51 | E | Small | |
| 19 | 52 | A | Large | |
| 20 | 52 | B | Small | |
| 21 | 52 | C | Small | |
| 22 | 52 | D | Large | |
| 23 | 52 | E | Small | |
| 24 | 2 | A | Large | |
| 25 | 2 | B | Small | |
| 26 | 2 | C | Large | |
| 27 | 2 | D | Large | |
| 28 | 2 | E | Small | |
| 29 | 3 | A | Large | |
| 30 | 3 | B | Small | |
| 31 | 2019 | 3 | C | Large |
| 32 | 3 | D | Large | |
| 33 | 3 | E | Small | |
| 34 | 3 | B | Large | |
| 35 | 3 | C | Small | |
| 36 | 4 | A | Large | |
| 37 | 4 | B | Small | |
| 38 | 4 | C | Small | |
| 39 | 4 | D | Large | |
| 40 | 4 | E | Small | |
| 41 | 4 | B | Large |
| Toxins | Precursor Ion | Product Ion | Frag | CE | CAV | Polarity |
|---|---|---|---|---|---|---|
| 45-OH-homo-YTX | 1171.5 | 1091.5 | 250 | 40 | 4 | Negative |
| 869.5 | 88 | |||||
| 45-OH-YTX | 1157.5 | 1077.5 | 240 | 38 | 4 | Negative |
| 871.5 | 86 | |||||
| Homo-YTX | 1155.48 | 1075.5 | 250 | 40 | 4 | Negative |
| 869.4 | 88 | |||||
| YTX | 1141.47 | 1061.5 | 240 | 38 | 4 | Negative |
| 855.4 | 86 | |||||
| PTX-1 | 892.5 | 821.5 | 175 | 28 | 2 | Positive |
| 213.2 | 44 | |||||
| PTX-2 | 876.5 | 823.5 | 175 | 28 | 2 | Positive |
| 213.2 | 44 | |||||
| AZA-1 | 842.5 | 824.5 | 206 | 32 | 2 | Positive |
| 806.5 | 44 | |||||
| AZA-2 | 856.5 | 838.5 | 213 | 36 | 4 | Positive |
| 820.5 | 44 | |||||
| AZA-3 | 828.5 | 810.5 | 216 | 32 | 2 | Positive |
| 792.5 | 44 | |||||
| OA/DTX-2 | 803.46 | 113.2 | 350 | 66 | 7 | Negative |
| 255.1 | 50 | |||||
| DTX-1 | 817.5 | 255.1 | 350 | 54 | 7 | Negative |
| 113 | 70 | |||||
| SPX-13 | 692.45 | 674.4 | 180 | 42 | 4 | Positive |
| 164.1 | 54 | |||||
| SPX-13,19 | 678.44 | 660.4 | 149 | 30 | 4 | Positive |
| 164.1 | 54 | |||||
| SPX-20G | 706.47 | 688.4 | 152 | 30 | 4 | Positive |
| 164.1 | 54 | |||||
| PnTX-G | 694.47 | 458.3 | 149 | 30 | 4 | Positive |
| 164.1 | 54 | |||||
| PnTX-E | 784.5 | 446.3 | 149 | 30 | 4 | Positive |
| 164.1 | 54 | |||||
| PnTX-D | 782.48 | 446.3 | 149 | 30 | 4 | Positive |
| 164.1 | 54 | |||||
| PnTX-F | 766.5 | 446.3 | 149 | 30 | 4 | Positive |
| 164.1 | 54 | |||||
| PnTX-B and C | 741.47 | 458.3 | 149 | 30 | 4 | Positive |
| 164.1 | 54 | |||||
| PnTX A | 712.44 | 458.3 | 149 | 30 | 4 | Positive |
| 164.1 | 54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero, P.; Miguéns, N.; Rodríguez, I.; Botana, L.M. LC–MS/MS Analysis of the Emerging Toxin Pinnatoxin-G and High Levels of Esterified OA Group Toxins in Galician Commercial Mussels. Toxins 2019, 11, 394. https://doi.org/10.3390/toxins11070394
Otero P, Miguéns N, Rodríguez I, Botana LM. LC–MS/MS Analysis of the Emerging Toxin Pinnatoxin-G and High Levels of Esterified OA Group Toxins in Galician Commercial Mussels. Toxins. 2019; 11(7):394. https://doi.org/10.3390/toxins11070394
Chicago/Turabian StyleOtero, Paz, Natalia Miguéns, Inés Rodríguez, and Luis M. Botana. 2019. "LC–MS/MS Analysis of the Emerging Toxin Pinnatoxin-G and High Levels of Esterified OA Group Toxins in Galician Commercial Mussels" Toxins 11, no. 7: 394. https://doi.org/10.3390/toxins11070394
APA StyleOtero, P., Miguéns, N., Rodríguez, I., & Botana, L. M. (2019). LC–MS/MS Analysis of the Emerging Toxin Pinnatoxin-G and High Levels of Esterified OA Group Toxins in Galician Commercial Mussels. Toxins, 11(7), 394. https://doi.org/10.3390/toxins11070394