Alteration of Bumblebee Venom Composition toward Higher Elevation
Abstract
:1. Introduction
2. Results
2.1. The Venom Composition of B. pascuorum Varies along the Elevation Gradient
2.2. Venom Toxins Show Differential Responses to Environmental Variations
2.3. Defensive Venom Toxins Respond Similarly along the Environmental Gradient
3. Discussion
4. Conclusions
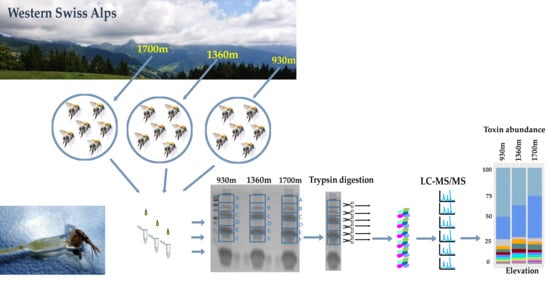
5. Materials and Methods
5.1. Study Area and Bumblebee Sampling
5.2. Venom Extraction and Protein Identification of Venom Components
5.3. Environmental Data
5.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calvete, J.J. Venomics: Digging into the evolution of venomous systems and learning to twist nature to fight pathology. J. Proteom. 2009, 72, 121–126. [Google Scholar] [CrossRef]
- Schmidt, J.O. Biochemistry of insect venoms. Annu. Rev. Entomol. 1982, 27, 339–368. [Google Scholar] [CrossRef]
- Whittington, C.M.; Belov, K. Tracing monotreme venom evolution in the genomics era. Toxins 2014, 6, 1260–1273. [Google Scholar] [CrossRef] [Green Version]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Arbuckle, K. Evolutionary Context of Venom in Animals. In Evolution of Venomous Animals and Their Toxins; Malhotra, A., Ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 3–31. [Google Scholar] [CrossRef]
- Fry, B.G.; Scheib, H.; van der Weerd, L.; Young, B.; McNaughtan, J.; Ramjan, S.F.; Vidal, N.; Poelmann, R.E.; Norman, J.A. Evolution of an arsenal: Structural and functional diversification of the venom system in the advanced snakes (Caenophidia). MCP 2008, 7, 215–246. [Google Scholar] [CrossRef] [Green Version]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K.; et al. Early evolution of the venom system in lizards and snakes. Nature 2006, 439, 584–588. [Google Scholar] [CrossRef]
- Fry, B.G.; Vidal, N.; van der Weerd, L.; Kochva, E.; Renjifo, C. Evolution and diversification of the Toxicofera reptile venom system. J. Proteom. 2009, 72, 127–136. [Google Scholar] [CrossRef]
- Zhang, Y. Why do we study animal toxins? Zool. Res. 2015, 36, 183–222. [Google Scholar] [CrossRef]
- Gibbs, H.L.; Rossiter, W. Rapid Evolution by Positive Selection and Gene Gain and Loss: PLA2 Venom Genes in Closely Related Sistrurus Rattlesnakes with Divergent Diets. J. Mol. Evol. 2008, 66, 151–166. [Google Scholar] [CrossRef]
- Westermann, F.L.; McPherson, I.S.; Jones, T.H.; Milicich, L.; Lester, P.J. Toxicity and utilization of chemical weapons: Does toxicity and venom utilization contribute to the formation of species communities? Ecol. Evol. 2015, 5, 3103–3113. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez de la Vega, R.C.; Schwartz, E.F.; Possani, L.D. Mining on scorpion venom biodiversity. Toxicon Off. J. Int. Soc. Toxinol. 2010, 56, 1155–1161. [Google Scholar] [CrossRef]
- Columbus-Shenkar, Y.Y.; Sachkova, M.Y.; Macrander, J.; Fridrich, A.; Modepalli, V.; Reitzel, A.M.; Sunagar, K.; Moran, Y. Dynamics of venom composition across a complex life cycle. eLife 2018, 7, e35014. [Google Scholar] [CrossRef]
- Gangur, A.N.; Smout, M.; Liddell, M.J.; Seymour, J.E.; Wilson, D.; Northfield, T.D. Changes in predator exposure, but not in diet, induce phenotypic plasticity in scorpion venom. Proc. Biol. Sci. 2017, 284, 20171364. [Google Scholar] [CrossRef] [Green Version]
- Holding, M.L.; Biardi, J.E.; Gibbs, H.L. Coevolution of venom function and venom resistance in a rattlesnake predator and its squirrel prey. Proc. Biol. Sci. 2016, 283, 20152814. [Google Scholar] [CrossRef]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Gibbs, H.L.; Mackessy, S.P. Functional basis of a molecular adaptation: Prey-specific toxic effects of venom from Sistrurus rattlesnakes. Toxicon Off. J. Int. Soc. Toxinol. 2009, 53, 672–679. [Google Scholar] [CrossRef]
- Gibbs, H.L.; Sanz, L.; Chiucchi, J.E.; Farrell, T.M.; Calvete, J.J. Proteomic analysis of ontogenetic and diet-related changes in venom composition of juvenile and adult Dusky Pigmy rattlesnakes (Sistrurus miliarius barbouri). J. Proteom. 2011, 74, 2169–2179. [Google Scholar] [CrossRef]
- O’Hara, E.P.; Caldwell, G.S.; Bythell, J. Equistatin and equinatoxin gene expression is influenced by environmental temperature in the sea anemone Actinia equina. Toxicon Off. J. Int. Soc. Toxinol. 2018, 153, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Strickland, J.L.; Smith, C.F.; Mason, A.J.; Schield, D.R.; Borja, M.; Castaneda-Gaytan, G.; Spencer, C.L.; Smith, L.L.; Trapaga, A.; Bouzid, N.M.; et al. Evidence for divergent patterns of local selection driving venom variation in Mojave Rattlesnakes (Crotalus scutulatus). Sci. Rep. 2018, 8, 17622. [Google Scholar] [CrossRef]
- Winter, K.L.; Isbister, G.K.; McGowan, S.; Konstantakopoulos, N.; Seymour, J.E.; Hodgson, W.C. A pharmacological and biochemical examination of the geographical variation of Chironex fleckeri venom. Toxicol. Lett. 2010, 192, 419–424. [Google Scholar] [CrossRef]
- Zancolli, G.; Calvete, J.J.; Cardwell, M.D.; Greene, H.W.; Hayes, W.K.; Hegarty, M.J.; Herrmann, H.-W.; Holycross, A.T.; Lannutti, D.I.; Mulley, J.F.; et al. When one phenotype is not enough: Divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. Proc. R. Soc. B 2019, 286, 20182735. [Google Scholar] [CrossRef] [Green Version]
- Huber, J.T. Biodiversity of Hymenoptera. In Insect Biodiversity; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 303–323. [Google Scholar] [CrossRef]
- Klopfstein, S.; Vilhelmsen, L.; Heraty, J.M.; Sharkey, M.; Ronquist, F. The Hymenopteran Tree of Life: Evidence from Protein-Coding Genes and Objectively Aligned Ribosomal Data. PLoS ONE 2013, 8, e69344. [Google Scholar] [CrossRef]
- Cameron, S.A.; Williams, P.H. Phylogeny of bumble bees in the New World subgenus Fervidobombus (Hymenoptera: Apidae): Congruence of molecular and morphological data. Mol. Phylogenetics Evol. 2003, 28, 552–563. [Google Scholar] [CrossRef]
- Heinrich, B. Resource heterogeneity and patterns of movement in foraging bumblebees. Oecologia 1979, 40, 235–245. [Google Scholar] [CrossRef]
- Pradervand, J.N.; Pellissier, L.; Rossier, L.; Dubuis, A.; Guisan, A.; Cherix, D. Diversity of bumblebees (Bombus Latreille, Apidae) in the Alps of the canton Vaud (Switzerland). Bull. Société Entomol. Suisse 2011, 84, 45–66. [Google Scholar]
- Woodard, S.H. Bumble bee ecophysiology: Integrating the changing environment and the organism. Curr. Opin. Insect Sci. 2017, 22, 101–108. [Google Scholar] [CrossRef]
- Williams, P.H.; Cameron, S.A.; Hines, H.M.; Cederberg, B.; Rasmont, P. A simplified subgeneric classification of the bumblebees (genus Bombus). Apidologie 2008, 39, 46–74. [Google Scholar] [CrossRef] [Green Version]
- Biella, P.; Bogliani, G.; Cornalba, M.; Manino, A.; Neumayer, J.; Porporato, M.; Rasmont, P.; Milanesi, P. Distribution patterns of the cold adapted bumblebee Bombus alpinus in the Alps and hints of an uphill shift (Insecta: Hymenoptera: Apidae). J. Insect Conserv. 2017, 21, 357–366. [Google Scholar] [CrossRef]
- Free, J. Insect Pollination of Crops; Academic Press: London, UK; New York, NY, USA, 1993; pp. 172–180. [Google Scholar]
- Dirnböck, T.; Essl, F.; Rabitsch, W. Disproportional risk for habitat loss of high-altitude endemic species under climate change. Glob. Chang. Biol. 2011, 17, 990–996. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Araújo, M.B.; Errea, M.P.; Martínez-Rica, J.P. Exposure of global mountain systems to climate warming during the 21st Century. Glob. Environ. Chang. 2007, 17, 420–428. [Google Scholar] [CrossRef]
- Von Reumont, B.M.; Campbell, L.I.; Jenner, R.A. Quo vadis venomics? A roadmap to neglected venomous invertebrates. Toxins 2014, 6, 3488–3551. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef] [Green Version]
- Barkan, N.P.; Bayazit, M.B.; Ozel Demiralp, D. Proteomic Characterization of the Venom of Five Bombus (Thoracobombus) Species. Toxins 2017, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Van Vaerenbergh, M.; Debyser, G.; Smagghe, G.; Devreese, B.; de Graaf, D.C. Unraveling the venom proteome of the bumblebee (Bombus terrestris) by integrating a combinatorial peptide ligand library approach with FT-ICR MS. Toxicon Off. J. Int. Soc. Toxinol. 2015, 102, 81–88. [Google Scholar] [CrossRef]
- Argiolas, A.; Pisano, J.J. Bombolitins, a new class of mast cell degranulating peptides from the venom of the bumblebee Megabombus pennsylvanicus. J. Biol. Chem. 1985, 260, 1437–1444. [Google Scholar]
- Schmidt, J.O. The Insect Sting Pain Scale: How the Pain and Lethality of Ant, Wasp, and Bee Venoms Can Guide the Way for Human Benefit. Preprints 2019, 2019050318. [Google Scholar]
- Goulson, D.; O’Connor, S.; Park, K.J. The impacts of predators and parasites on wild bumblebee colonies. Ecol. Entomol. 2018, 43, 168–181. [Google Scholar] [CrossRef]
- Holding, M.L.; Margres, M.J.; Rokyta, D.R.; Gibbs, H.L. Local prey community composition and genetic distance predict venom divergence among populations of the northern Pacific rattlesnake (Crotalus oreganus). J. Evol. Biol. 2018, 31, 1513–1528. [Google Scholar] [CrossRef]
- Junior, R.S.F.; Sciani, J.M.; Marques-Porto, R.; Junior, A.L.; Orsi, R.d.O.; Barraviera, B.; Pimenta, D.C. Africanized honey bee (Apis mellifera) venom profiling: Seasonal variation of melittin and phospholipase A 2 levels. Toxicon Off. J. Int. Soc. Toxinol. 2010, 56, 355–362. [Google Scholar] [CrossRef]
- Romero, G.Q.; Gonçalves-Souza, T.; Kratina, P.; Marino, N.A.C.; Petry, W.K.; Sobral-Souza, T.; Roslin, T. Global predation pressure redistribution under future climate change. Nat. Clim. Chang. 2018, 8, 1087–1091. [Google Scholar] [CrossRef]
- Pennings, S.C.; Silliman, B.R. Linking Biogeography and Community Ecology: Latitudinal Variation in Plant-Herbivore Interaction Strength. Ecology 2005, 86, 2310–2319. [Google Scholar] [CrossRef]
- Roslin, T.; Hardwick, B.; Novotny, V.; Petry, W.K.; Andrew, N.R.; Asmus, A.; Barrio, I.C.; Basset, Y.; Boesing, A.L.; Bonebrake, T.C.; et al. Higher predation risk for insect prey at low latitudes and elevations. Science 2017, 356, 742–744. [Google Scholar] [CrossRef] [Green Version]
- Morgenstern, D.; King, G.F. The venom optimization hypothesis revisited. Toxicon Off. J. Int. Soc. Toxinol. 2013, 63, 120–128. [Google Scholar] [CrossRef]
- Goulson, D.; Lye, G.C.; Darvill, B. Decline and conservation of bumble bees. Annu. Rev. Entomol. 2008, 53, 191–208. [Google Scholar] [CrossRef]
- Williams, P.; Colla, S.; Xie, Z. Bumblebee Vulnerability: Common Correlates of Winners and Losers across Three Continents. Conserv. Biol. 2009, 23, 931–940. [Google Scholar] [CrossRef]
- Clapp, L.E.; Klette, K.L.; DeCoster, M.A.; Bernton, E.; Petras, J.M.; Dave, J.R.; Laskosky, M.S.; Smallridge, R.C.; Tortella, F.C. Phospholipase A2-induced neurotoxicity in vitro and in vivo in rats. Brain Res. 1995, 693, 101–111. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Bee Venom Phospholipase A2: Yesterday’s Enemy Becomes Today’s Friend. Toxins 2016, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Perez-Riverol, A.; Lasa, A.M.; Dos Santos-Pinto, J.R.A.; Palma, M.S. Insect venom phospholipases A1 and A2: Roles in the envenoming process and allergy. Insect Biochem. Mol. Biol. 2019, 105, 10–24. [Google Scholar] [CrossRef]
- Lozano, R.M.; Yee, B.C.; Buchanan, B.B. Thioredoxin-linked reductive inactivation of venom neurotoxins. Arch. Biochem. Biophys. 1994, 309, 356–362. [Google Scholar] [CrossRef]
- Winningham, K.M.; Fitch, C.D.; Schmidt, M.; Hoffman, D.R. Hymenoptera venom protease allergens. J. Allergy Clin. Immunol. 2004, 114, 928–933. [Google Scholar] [CrossRef]
- Brodie, E.D., Jr.; Ridenhour, B.J.; Brodie, E.D., III. The evolutionary response of predators to dangerous prey: Hotspots and coldspots in the geographic mosaic of coevolution between garter snakes and newts. Evol. Int. J. Org. Evol. 2002, 56, 2067–2082. [Google Scholar] [CrossRef]
- Hanifin, C.T.; Brodie, E.D.; Brodie, E.D. Phenotypic mismatches reveal escape from arms-race coevolution. PLoS Biol. 2008, 6, e60. [Google Scholar] [CrossRef]
- Pekar, S.; Liznarova, E.; Bocanek, O.; Zdrahal, Z. Venom of prey-specialized spiders is more toxic to their preferred prey: A result of prey-specific toxins. J. Anim. Ecol. 2018, 87, 1639–1652. [Google Scholar] [CrossRef]
- Dobzhansky, T. Evolution in the tropics. Am. Sci. 1950, 38, 209–221. [Google Scholar]
- MacArthur, R.H. Geographical Ecology: Patterns in the Distribution of Species; Princeton University Press: Princeton, NJ, USA, 1972. [Google Scholar]
- Pellissier, L.; Fiedler, K.; Ndribe, C.; Dubuis, A.; Pradervand, J.-N.; Guisan, A.; Rasmann, S. Shifts in species richness, herbivore specialization, and plant resistance along elevation gradients. Ecol. Evol. 2012, 2, 1818–1825. [Google Scholar] [CrossRef]
- Descombes, P.; Marchon, J.; Pradervand, J.-N.; Bilat, J.; Guisan, A.; Rasmann, S.; Pellissier, L. Community-level plant palatability increases with elevation as insect herbivore abundance declines. J. Ecol. 2017, 105, 142–151. [Google Scholar] [CrossRef]
- RECHALP: A Geodatabase of Scientific Metadata for the Vaud Alps. Available online: http://rechalp.unil.ch (accessed on 11 November 2019).
- NCBI Protein Blast. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 18 December 2019).
- Broennimann, O. CHclim25: A High Spatial and Temporal Resolution Climate Dataset for Switzerland; Ecospat laboratory, University of Lausanne: Lausanne, Switzerland, 2018. [Google Scholar]
- Jolliffe, I. Principal Component Analysis. In International Encyclopedia of Statistical Science; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1094–1096. [Google Scholar] [CrossRef]
- Dray, D. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 18 December 2019).
- Jain, A.K.; Dubes, R.C. Algorithms for Clustering Data; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1988; p. 320. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkan, N.P.; Chevalier, M.; Pradervand, J.-N.; Guisan, A. Alteration of Bumblebee Venom Composition toward Higher Elevation. Toxins 2020, 12, 4. https://doi.org/10.3390/toxins12010004
Barkan NP, Chevalier M, Pradervand J-N, Guisan A. Alteration of Bumblebee Venom Composition toward Higher Elevation. Toxins. 2020; 12(1):4. https://doi.org/10.3390/toxins12010004
Chicago/Turabian StyleBarkan, Nezahat Pınar, Mathieu Chevalier, Jean-Nicolas Pradervand, and Antoine Guisan. 2020. "Alteration of Bumblebee Venom Composition toward Higher Elevation" Toxins 12, no. 1: 4. https://doi.org/10.3390/toxins12010004
APA StyleBarkan, N. P., Chevalier, M., Pradervand, J.-N., & Guisan, A. (2020). Alteration of Bumblebee Venom Composition toward Higher Elevation. Toxins, 12(1), 4. https://doi.org/10.3390/toxins12010004