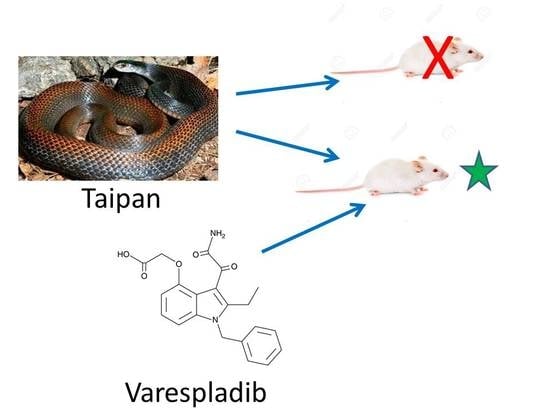
Varespladib (LY315920) and Methyl Varespladib (LY333013) Abrogate or Delay Lethality Induced by Presynaptically Acting Neurotoxic Snake Venoms
Abstract
:1. Introduction
2. Results
2.1. Estimation of LD50s
2.2. Rescue Experiments
2.3. Reversion of Paralytic Effects
2.4. Effect of Neostigmine
3. Discussion
4. Materials and Methods
4.1. Venoms
4.2. Drugs
4.3. Rescue Experiments and Observations on Lethality
4.3.1. Estimation of Subcutaneous Median Lethal Dose (LD50)
4.3.2. Rescue Experiments
4.3.3. Reversal of Paralytic Effects
4.4. Inhibitory Effect of Neostigmine
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17079. [Google Scholar] [CrossRef]
- World Health Organization. Snakebite Envenoming, A Strategy for Prevention and Control; World Health Organization: Geneva, Switzerland, 2019; pp. 1–50. [Google Scholar]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B.; León, G.; Rucavado, A.; Chaves, F.; Angulo, Y. Trends in snakebite envenomation therapy: Scientific, technological and public health considerations. Curr. Pharm. Des. 2007, 13, 2935–2950. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Escalante, T.; Franceschi, A.; Chaves, F.; León, G.; Cury, Y.; Ovadia, M.; Gutiérrez, J.M. Inhibition of local hemorrhage and dermonecrosis induced by Bothrops asper snake venom: Effectiveness of early in situ administration of the peptidomimetic metalloproteinase inhibitor batimastat and the chelating agent CaNa2DTA. Am. J. Trop. Med. Hyg. 2000, 63, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, A.S.; Rucavado, A.; Gutiérrez, J.M. Peptidomimetic hydroxamate metalloproteinase inhibitors abrogate local and systemic toxicity induced by Echis ocellatus (saw-scaled) snake venom. Toxicon 2017, 132, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.; Samuel, S.; Merkel, J.; Bickler, P. Varespladib (LY315920) appears to be a potent, broad-spectrum, inhibitor of snake venom phospholipase A2 and a possible pre-referral treatment for envenomation. Toxins 2016, 8, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.J.; Tsai, C.Y.; Hu, W.P.; Chang, L.S. DNA aptamers against Taiwan banded krait α-bungarotoxin recognize Taiwan cobra cardiotoxins. Toxins 2016, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.; Lee, S.H.; Gutiérrez, J.M.; Shea, K.J. Engineered nanoparticles bind elapid snake venom toxins and inhibit venom-induced dermonecrosis. PLoS Negl. Trop. Dis. 2018, 12, e0006736. [Google Scholar] [CrossRef]
- Albulescu, L.O.; Kazandjian, T.; Slagboom, J.; Bruyneel, B.; Ainsworth, S.; Alsolaiss, J.; Wagstaff, S.C.; Whiteley, G.; Harrison, R.A.; Ulens, C.; et al. A decoy-receptor approach using nicotinic acetylcholine receptor mimics reveals their potential as novel therapeutics against neurotoxic snakebite. Front. Pharmacol. 2019, 10, 848. [Google Scholar] [CrossRef] [Green Version]
- Adis, R.; Profile, D. Varespladib. Am. J. Cardiovasc. Drugs 2011, 11, 137–143. [Google Scholar]
- Nicholls, S.J.; Kastelein, J.P.; Schwartz, G.G.; Bash, D.; Rosenson, R.S.; Cavender, M.A.; Brennan, M.S.; Koenig, W.; Jukema, W.; Nambi, V.; et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome. The VISTA-16 randomized clinical trial. JAMA 2014, 311, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Sanz, L.; Cid, P.; de la Torre, P.; Flores-Díaz, M.; dos Santos, M.C.; Borges, A.; Bremo, A.; Angulo, Y.; Lomonte, B.; et al. Snake venomics of the Central American rattlesnake Crotalus simus and the South American Crotalus durissus complex points to neurotoxicity as an adaptive paedomorphic trend along Crotalus dispersal in South America. J. Proteome Res. 2010, 9, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Fernández, J.; Vargas, M.; Villalta, M.; Segura, Á.; León, G.; Angulo, Y.; Paiva, O.; Matainaho, T.; Jensen, S.D.; et al. Comparative proteomic analysis of the venom of the taipan snake, Oxyuranus scutellatus, from Papua New Guinea and Australia: Role of neurotoxic and procoagulant effects in venom toxicity. J. Proteom. 2012, 75, 2128–2140. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Fernández, J.; Sanz, L.; Angulo, Y.; Sasa, M.; Gutiérrez, J.M.; Calvete, J.J. Venomous snakes of Costa Rica: Biological and medical implications of their venom proteomic profiles analyzed through the strategy of snake venomics. J. Proteom. 2014, 105, 323–339. [Google Scholar] [CrossRef]
- Tasoulis, T.; Isbister, G.F. A review and database of snake venom proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [Green Version]
- Lewin, M.R.; Gutiérrez, J.M.; Samuel, S.P.; Herrera, M.; Bryan-Quirós, W.; Lomonte, B.; Bickler, P.E.; Bulfone, T.C.; Williams, D.J. Delayed oral LY333013 rescues mice from highly neurotoxic, lethal doses of Papuan Taipan (Oxyuranus scutellatus) venom. Toxins 2018, 10, E380. [Google Scholar] [CrossRef] [Green Version]
- Lewin, M.R.; Gilliam, L.L.; Gilliam, J.; Samuel, S.P.; Bulfone, T.C.; Bickler, P.E.; Gutiérrez, J.M. Delayed LY333013 (oral) and Ly315920 (intravenous) reverse severe neurotoxicity and rescue juvenile pigs from lethal doses of Micrurus fulvius (Eastern coral snake) venom. Toxins 2018, 10, E479. [Google Scholar] [CrossRef] [Green Version]
- Bryan-Quirós, W.; Fernández, J.; Gutiérrez, J.M.; Lewin, M.R.; Lomonte, B. Neutralizing properties of LY315920 toward snake venom group I and II myotoxic phospholipases A2. Toxicon 2019, 157, 1–7. [Google Scholar] [CrossRef]
- Salvador, G.H.M.; Gomes, A.A.S.; Bryan-Quirós, W.; Fernández, J.; Lewin, M.R.; Gutiérrez, J.M.; Lomonte, B.; Fontes, M.R.M. Structural basis for phospholipase A2-like toxin inhibition by the synthetic compound Varespladib (LY315920). Sci. Rep. 2019, 9, 17203. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; Zdenek, C.N.; Op den Brouw, B.; Youngman, N.J.; Dobson, J.S.; Naude, A.; Vonk, F.J.; Fry, B.G. Coagulotoxic cobras: Clinical implications of strong anticoagulant actions of African spitting Naja venoms that are not neutralized by antivenom but are by LY315920 (Varespladib). Toxins 2018, 10, 516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, M.; Collaço, R.C.O.; Villalta, M.; Segura, Á.; Vargas, M.; Wright, C.E.; Paiva, O.; Matainaho, T.; Jensen, S.D.; León, G.; et al. Neutralization of the neuromuscular inhibition of venom and taipoxin from the taipan (Oxyuranus scutellatus) by F(ab’)2 and whole IgG antivenoms. Toxicol. Lett. 2016, 241, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Bulfone, T.C.; Samuel, S.P.; Bickler, P.E.; Lewin, M.R. Developing small molecule therapeutics for the initial and adjunctive treatment of snakebite. J. Trop. Med. 2018, 4320175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, E.; Eaker, D.; Rydén, L. Purification of a neurotoxin from the venom of the Australian tiger snake Notechis scutatus. Toxicon 1972, 10, 405–413. [Google Scholar] [CrossRef]
- Halpert, J.; Eaker, D. Amino acid sequence of a presynaptic neurotoxin from the venom of Notechis scutatus scutatus (Australian tiger snake). J. Biol. Chem. 1975, 250, 6990–6997. [Google Scholar] [PubMed]
- Harris, J.B.; Grubb, B.D.; Maltin, C.A.; Dixon, R. The neurotoxicity of the venom phospholipases A2 notexin and taipoxin. Exp. Neurol. 2000, 161, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, K.; Narita, K.; Lee, C.Y. Amino acid sequences of the two polypeptide chains of β1-bungarotoxin from the venom of Bungarus multicinctus. J. Biochem. 1978, 83, 101–115. [Google Scholar] [CrossRef]
- Dixon, R.W.; Harris, J.B. Nerve terminal damage by β-bungarotoxin: Its clinical significance. Am. J. Pathol. 1999, 154, 447–455. [Google Scholar] [CrossRef]
- Sunegar, K.; Jackson, T.N.W.; Reeks, T.; Fry, B.G. Group I phospholipase A2 enzymes. In Venomous Reptiles and Their Toxins. Evolution, Pathophysiology & Biodiscovery; Fry, B.G., Ed.; Oxford University Press: Oxford, UK, 2015; pp. 327–334. [Google Scholar]
- Faure, G.; Xu, H.; Saul, F.A. Crystal structure of crotoxin reveals key residues involved in the stability and toxicity of this potent heterodimeric β-neurotoxin. J. Mol. Biol. 2011, 412, 176–191. [Google Scholar] [CrossRef]
- Gopalakrishnakone, P.; Hawgood, B.J. Morphological changes induced by crotoxin in murine nerve and neuromuscular junction. Toxicon 1984, 22, 791–804. [Google Scholar] [CrossRef]
- Fohlman, J.; Eaker, D.; Karlsson, E.; Thesleff, S. Taipoxin, an extremely ptent presynaptic neurotoxin from the venom of the Australian snake taipan (Oxyuranus s. scutellatus). Isolation, characterization, quaternary structure and pharmacological properties. Eur. J. Biochem. 1976, 68, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Halpert, J.; Fohlman, J.; Eaker, D. Amino acid sequence of a postsynaptic neurotoxin from the venom of the Australian tiger snake Notechis scutatus scutatus. Biochimie 1979, 61, 719–723. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y.; Tan, N.H. Revisiting Notechis scutatus venom: On shotgun proteomics and neutralization by the “bivalent” Sea Snake Antivenom. J. Proteom. 2016, 144, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Ranawaka, U.K.; Lalloo, D.G.; de Silva, H.J. Neurotoxicity in snakebite. The limits of our knowledge. PLoS Negl. Trop. Dis. 2013, 7, e2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montecucco, C.; Gutiérrez, J.M.; Lomonte, B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: Common aspects of their mechanisms of action. Cell. Mol. Life Sci. 2008, 65, 2897–2912. [Google Scholar] [CrossRef]
- Šribar, J.; Oberčkal, J.; Križaj, I. Understanding the molecular mechanisms underlying the presynaptic toxicity of secreted phospholipases A2: An update. Toxicon 2014, 89, 9–16. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]



| Treatment | Deaths/Total at 3 h |
|---|---|
| Venom + PBS | 4/4 |
| Venom + VAR (0 min) | 4/4 |
| Venom + VAR (60 min) | 4/4 |
| Venom + VAR (0 min and 60 min) | 4/4 |
| Treatment | Deaths/Total at 3 h | Deaths/Total at 6 h | Deaths/Total at 24 h |
|---|---|---|---|
| Venom + PBS | 4/4 | ||
| Venom + VAR (0 min) | 0/4 | 0/4 | 4/4 |
| Venom + VAR (60 min) | 0/4 | 0/4 | 4/4 |
| Venom + VAR (0 min and 240 min) | 0/4 | 0/4 | 4/4 |
| Venom + VAR (60 min and 240 min) | 0/4 | 0/4 | 4/4 |
| Treatment | Deaths/Total at 3 h | Deaths/Total at 6 h | Deaths/Total at 24 h |
|---|---|---|---|
| Venom + PBS | 4/4 | ||
| Venom + VAR (0 min) | 0/4 | ¾ | 4/4 |
| Venom + VAR (60 min) | 0/4 | 0/4 | 4/4 |
| Venom + VAR (0 min and 240 min) | 0/4 | ¾ | 4/4 |
| Venom + VAR (60 min and 240 min) | 0/4 | 0/4 | 4/4 |
| Treatment | Deaths/Total at 3 h | Deaths/Total at 6 h | Deaths/Total at 24 h |
|---|---|---|---|
| Venom + PBS | 4/4 | ||
| Venom + VAR (0 min) | 0/4 | 0/4 | 4/4 |
| Venom + VAR (60 min) | 0/4 | 0/4 | 4/4 |
| Venom + VAR (0 min and 240 min) | 0/4 | 0/4 | 4/4 |
| Venom + VAR (60 min and 240 min) | 0/4 | 0/4 | 4/4 |
| Treatment | Deaths/Total at 3 h | Deaths/Total at 6 h | Deaths/Total at 24 h |
|---|---|---|---|
| Venom + PBS | 4/4 | ||
| Venom + VAR (0 min) | 0/4 | 0/4 | 0/4 |
| Venom + VAR (30 min) | 0/4 | 0/4 | 0/4 |
| Venom + VAR (60 min) | 0/4 | 0/4 | 1/4 (0/4) b |
| Venom + VAR (90 min) | 0/4 | 0/4 | 4/4 |
| Venom + VAR (0 min and 240 min) | 0/4 | 0/4 | 0/4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, J.M.; Lewin, M.R.; Williams, D.J.; Lomonte, B. Varespladib (LY315920) and Methyl Varespladib (LY333013) Abrogate or Delay Lethality Induced by Presynaptically Acting Neurotoxic Snake Venoms. Toxins 2020, 12, 131. https://doi.org/10.3390/toxins12020131
Gutiérrez JM, Lewin MR, Williams DJ, Lomonte B. Varespladib (LY315920) and Methyl Varespladib (LY333013) Abrogate or Delay Lethality Induced by Presynaptically Acting Neurotoxic Snake Venoms. Toxins. 2020; 12(2):131. https://doi.org/10.3390/toxins12020131
Chicago/Turabian StyleGutiérrez, José María, Matthew R. Lewin, David. J. Williams, and Bruno Lomonte. 2020. "Varespladib (LY315920) and Methyl Varespladib (LY333013) Abrogate or Delay Lethality Induced by Presynaptically Acting Neurotoxic Snake Venoms" Toxins 12, no. 2: 131. https://doi.org/10.3390/toxins12020131
APA StyleGutiérrez, J. M., Lewin, M. R., Williams, D. J., & Lomonte, B. (2020). Varespladib (LY315920) and Methyl Varespladib (LY333013) Abrogate or Delay Lethality Induced by Presynaptically Acting Neurotoxic Snake Venoms. Toxins, 12(2), 131. https://doi.org/10.3390/toxins12020131