Ochratoxin A in Beers Marketed in Portugal: Occurrence and Human Risk Assessment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Validation and Quality Control
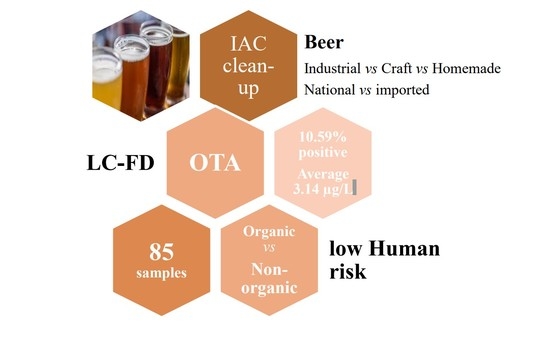
2.2. Frequency and Occurrence of OTA in Beer Samples
2.3. Human Estimated Daily Intake and Risk Assessment
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Sampling and Sample Characterization
4.3. Experimental Procedure
4.4. Validation and Quality Control Assays
4.5. Calculation of the Human Estimated Daily Intake and Risk Assessment
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019, 0, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.B.; Fernández-Franzón, M.; Ruiz, M.J.; Juan-García, A. Presence of ochratoxin a (OTA) mycotoxin in alcoholic drinks from southern european countries: Wine and beer. J. Agric. Food Chem. 2014, 62, 7643–7651. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 years of research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [Green Version]
- Duarte, S.C.; Pena, A.; Lino, C.M. Human ochratoxin A biomarkers-from exposure to effect. Crit. Rev. Toxicol. 2011, 41, 187–212. [Google Scholar] [CrossRef] [PubMed]
- Gifford, F.J.; Gifford, R.M.; Eddleston, M.; Dhaun, N. Endemic Nephropathy around the World. Kidney Int. Rep. 2017, 2, 282–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadot, I.; Declèves, A.E.; Nortier, J.; Caron, N. An integrated view of aristolochic acid nephropathy: Update of the literature. Int. J. Mol. Sci. 2017, 18, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Aresta, A.; Palmisano, F.; Vatinno, R.; Zambonin, C.G. Ochratoxin A determination in beer by solid-phase microextraction coupled to liquid chromatography with fluorescence detection: A fast and sensitive method for assessment of noncompliance to legal limits. J. Agric. Food Chem. 2006, 54, 1594–1598. [Google Scholar] [CrossRef]
- Juan, C.; Mañes, J.; Font, G.; Juan-García, A. Determination of mycotoxins in fruit berry by-products using QuEChERS extraction method. LWT Food Sci. Technol. 2017, 86, 344–351. [Google Scholar] [CrossRef]
- Juan, C.; Moltó, J.C.; Lino, C.M.; Mañes, J. Determination of ochratoxin A in organic and non-organic cereals and cereal products from Spain and Portugal. Food Chem. 2008, 107, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Anli, E.; Alkis, İ.M. Ochratoxin A and Brewing Technology: A Review. J. Inst. Brew. 2010, 116, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Běláková, S.; Benešová, K.; Mikulíková, R.; Svoboda, Z. Determination of ochratoxin A in brewing materials and beer by ultra performance liquid chromatography with fluorescence detection. Food Chem. 2011, 126, 321–325. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Rastelli, S.; Mulazzi, A.; Donadini, G.; Pietri, A. Mycotoxin occurrence in beer produced in several European countries. Food Control 2011, 22, 2059–2064. [Google Scholar] [CrossRef]
- Kawashima, L.M.; Vieira, A.P.; Valente Soares, L.M. Fumonisin B 1 and ochratoxin A in beers made in Brazil Fumonisina B 1 e ocratoxina A em cervejas fabricadas no Brasil. Ciência E Tecnol. Aliment. 2007, 27, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Medina, Á.; Jiménez, M.; Gimeno-Adelantado, J.V.; Valle-Algarra, F.M.; Mateo, R. Determination of ochratoxin A in beer marketed in Spain by liquid chromatography with fluorescence detection using lead hydroxyacetate as a clean-up agent. J. Chromatogr. A 2005, 1083, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Chung, S.H.; Kim, Y.B. Ochratoxin a in Korean food commodities: Occurrence and safety evaluation. J. Agric. Food Chem. 2005, 53, 4637–4642. [Google Scholar] [CrossRef] [PubMed]
- Tangni, E.K.; Ponchaut, S.; Maudoux, M.; Rozenberg, R.; Larondelle, Y. Ochratoxin A in domestic and imported beers in Belgium: Occurence and exposure assessment. Food Addit. Contam. 2002, 19, 1169–1179. [Google Scholar] [CrossRef]
- Odhav, B.; Naicker, V. Mycotoxins in South African traditionally brewed beers. Food Addit. Contam. 2002, 19, 55–61. [Google Scholar] [CrossRef]
- Lhotská, I.; Šatínský, D.; Havlíková, L.; Solich, P. A fully automated and fast method using direct sample injection combined with fused-core column on-line SPE-HPLC for determination of ochratoxin A and citrinin in lager beers. Anal. Bioanal. Chem. 2016, 408, 3319–3329. [Google Scholar] [CrossRef]
- Rubert, J.; Soler, C.; Marín, R.; James, K.J.; Mañes, J. Mass spectrometry strategies for mycotoxins analysis in European beers. Food Control 2013, 30, 122–128. [Google Scholar] [CrossRef]
- Prelle, A.; Spadaro, D.; Denca, A.; Garibaldi, A.; Gullino, M.L. Comparison of clean-up methods for ochratoxin a on wine, beer, roasted coffee and chili commercialized in Italy. Toxins 2013, 5, 1827–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdavi, R.; Khorrami, S.A.H.; Jabbari, V. Evaluation of ochratoxin A contamination in non alcoholic beers in Iran. Res. J. Biol. Sci. 2007, 2, 546–550. [Google Scholar]
- Kabak, B. Ochratoxin A in cereal-derived products in Turkey: Occurrence and exposure assessment. Food Chem. Toxicol. 2009, 47, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tan, Y.; Wang, Y.; Xu, R. Occurrence of ochratoxin a in wine and beer samples from China. Food Addit. Contam. B Surveill. 2011, 4, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Uyama, A.; Mochizuki, N. Development of a Multi-mycotoxin Analysis in Beer-based Drinks by a Modified QuEChERS Method and Ultra-High-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry. Anal. Sci. 2011, 27, 629–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinsch, M.; Töpfer, A.; Lehmann, A.; Nehls, I.; Panne, U. Determination of ochratoxin A in beer by LC-MS/MS ion trap detection. Food Chem. 2007, 100, 312–317. [Google Scholar] [CrossRef]
- Novo, P.; Moulas, G.; França Prazeres, D.M.; Chu, V.; Conde, J.P. Detection of ochratoxin A in wine and beer by chemiluminescence-based ELISA in microfluidics with integrated photodiodes. Sens. Actuators B Chem. 2013, 176, 232–240. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Dofkova, M.; Skarkova, J.; Pfohl-Leszkowicz, A.; Ruprich, J. Ochratoxin a dietary exposure of ten population groups in the czech republic: Comparison with data over the world. Toxins 2015, 7, 3608–3635. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Regulation (EC) No 1881/2006. Off. J. Eur. Union 2006, 49, 5–24. [Google Scholar]
- EFSA. Opinion of the Scientific Panel on Contaminants in the food Chain on a Request from the Commission Related to Ochratoxin A in Food. Efsa J. 2006, 365, 1–56. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, L70, 12–34. [Google Scholar]
- Harcz, P.; Tangni, E.K.; Wilmart, O.; Moons, E.; Van Peteghem, C.; De Saeger, S.; Schneider, Y.J.; Larondelle, Y.; Pussemier, L. Intake of ochratoxin A and deoxynivalenol through beer consumption in Belgium. Food Addit. Contam. 2007, 24, 910–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascari, X.; Ramos, A.J.; Marín, S.; Sanchís, V. Mycotoxins and beer. Impact of beer production process on mycotoxin contamination. A review. Food Res. Int. 2018, 103, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo, R.; Medina, Á.; Mateo, E.M.; Mateo, F.; Jiménez, M. An overview of ochratoxin A in beer and wine. Int. J. Food Microbiol. 2007, 119, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Mastanjević, K.; Šarkanj, B.; Krska, R.; Sulyok, M.; Warth, B.; Mastanjević, K.; Šantek, B.; Krstanović, V. From malt to wheat beer: A comprehensive multi-toxin screening, transfer assessment and its influence on basic fermentation parameters. Food Chem. 2018, 254, 115–121. [Google Scholar] [CrossRef]
- Peters, J.; Van Dam, R.; Van Doorn, R.; Katerere, D.; Berthiller, F.; Haasnoot, W.; Nielen, M.W.F. Mycotoxin profiling of 1000 beer samples with a special focus on craft beer. PLoS ONE 2017, 12, e0185887. [Google Scholar] [CrossRef] [Green Version]
- Bertuzzi, T.; Rastelli, S.; Mulazzi, A.; Donadini, G.; Pietri, A. Known and Emerging Mycotoxins in Small- and Large-Scale Brewed Beer. Beverages 2018, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.P.; Jager, G.; Zyl, H.; Van Voss, H.; Hogg, T.; Graaf, C.; De Patricia, A.; Jager, G.; Zyl, H.; Van Voss, H. Cheers, proost, saúde: Cultural, contextual and psychological factors of wine and beer consumption in Portugal and in the Netherlands. Crit. Rev. Food Sci. Nutr. 2017, 57, 1340–1349. [Google Scholar] [CrossRef]
- The Brewers of Europe. The Contribution Made by Beer to the European Economy; The Brewers of Europe: Brussels, Belgium, 2016. [Google Scholar]
- De Nijs, M.; Mengelers, M.J.B.; Boon, P.E.; Heyndrickx, E.; Hoogenboom, L.A.P.; Lopez, P.; Mol, H.G.J. Strategies for estimating human exposure to mycotoxins via food. World Mycotoxin J. 2016, 9, 831–845. [Google Scholar] [CrossRef] [Green Version]
- Anselme, M.; Tangni, E.K.; Pussemier, L.; Motte, J.C.; Van Hove, F.; Schneider, Y.J.; Van Peteghem, C.; Larondelle, Y. Comparison of ochratoxin A and deoxynivalenol in organically and conventionally produced beers sold on the Belgian market. Food Addit. Contam. 2006, 23, 910–918. [Google Scholar] [CrossRef]
- IPCS. Dietary exposure assessment of chemicals in food. In Principles and Methods for the Risk Assessment of Chemicals in Food; International Programme on Chemical Safety; WHO: Geneve, Switzerland, 2009; p. 98. [Google Scholar]
- INE Statistics Portugal. Balança Alimentar Portuguesa 2012–2016; INE Statistics Portugal: Lisbon, Portugal, 2017. [Google Scholar]
- Arezes, P.M.; Barroso, M.P.; Cordeiro, P.; Costa, L.G.; Miguel, A.S. Estudo Antropométrico da População Portuguesa, 1st ed.; Instituto para a Segurança, Higiene e Saúde no Trabalho: Lisboa, Portugal, 2006. [Google Scholar]
| Spiking Level (µg/L) | Recovery (%) | RSD within-day (%) | RSD between-day (%) |
|---|---|---|---|
| 0.5 | 81.0 | 2.43 | 2.74 |
| 1 | 86.0 | 2.36 | 5.26 |
| 2 | 85.0 | 1.72 | 6.25 |
| Sample | Production | Type | Color | Type of Cereal | Alcohol Content (%) | Origin | Contamination (µg/L) |
| 17 | Craft beer | Ale | Pale | Barley and wheat malt | 4.7 | Portugal | 1.81 |
| 20 | Craft beer | Lager | Pale | Barley and wheat malt | 5.1 | Portugal | 0.5 |
| 22 | Craft beer | Lager | Pale | Barley malt, maize | 6 | Portugal | 0.54 |
| 31 | Homemade | Ale | Pale | Barley malt | 4.2 | New Zealand | 9.21 |
| 32 | Homemade | Ale | Pale | Barley malt | 5.6 | New Zealand | 11.25 |
| 6 | Industrial | Lager | Pale | Barley malt, maize, barley | 5 | Portugal | <LOQ |
| 18 | Industrial | Lager | Pale | Barley malt | 5 | Portugal | 1.81 |
| 19 | Industrial | Ale | Pale | Barley malt | 8.5 | Belgium | 1.74 |
| 21 | Industrial | Lager | Pale | Barley malt, maize, barley | 5.2 | Portugal | 1.22 |
| Total | |||||||
| Frequency (%) | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 10.6 |
| Range (µg/L) | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | <LOQ‒11.25 |
| Mean ± SD (µg/L) | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 3.14 ± 4.09 |
| Median | 1.74 | ||||||
| Category | Frequency Positive (total) | Mean ± SD | Median | |
|---|---|---|---|---|
| Production | Industrial | 44.4 (4.7) | 1.25 ± 0.74 | 1.48 |
| Craft | 33.3 (3.5) | 0.95 ± 0.66 | 0.54 | |
| Homemade | 22.2 (2.4) | 10.23 ± 1.44 | 10.23 | |
| Type of beer | Lager | 55.5 (5.9) | 0.86 ± 0.65 | 0.54 |
| Ale | 44.4 (4.7) | 6.00 ± 4.95 | 3 | |
| Alcohol (%) | <6% | 77.8 (8.2) | 3.72 ± 4.53 | 1.81 |
| ≥6% | 22.2 (2.4) | 1.14 ± 0.85 | 1.14 | |
| Type of cereal | Barley | 44.4 (4.7) | 6.00 ± 4.95 | 5.51 |
| Mixture | 55.5 (5.9) | 0.86 ± 0.65 | 0.54 | |
| Origin | Portugal | 66.7 (7.1) | 1.02 ± 0.70 | 0.88 |
| Abroad | 33.3 (3.5) | 7.40 ± 5.00 | 9.21 |
| Scenarios | EDI (ng/kg b.w./day) | EWI (ng/kg b.w./week) | EWI/TWI d (%) |
|---|---|---|---|
| I a | 0.67 | 4.71 | 3.92 |
| II b | 6.35 | 44.48 | 37.06 |
| III c | 22.74 | 159.16 | 132.63 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.J.G.; Teixeira, A.C.; Pereira, A.M.P.T.; Pena, A.; Lino, C.M. Ochratoxin A in Beers Marketed in Portugal: Occurrence and Human Risk Assessment. Toxins 2020, 12, 249. https://doi.org/10.3390/toxins12040249
Silva LJG, Teixeira AC, Pereira AMPT, Pena A, Lino CM. Ochratoxin A in Beers Marketed in Portugal: Occurrence and Human Risk Assessment. Toxins. 2020; 12(4):249. https://doi.org/10.3390/toxins12040249
Chicago/Turabian StyleSilva, Liliana J. G., Ana C. Teixeira, André M. P. T. Pereira, Angelina Pena, and Celeste M. Lino. 2020. "Ochratoxin A in Beers Marketed in Portugal: Occurrence and Human Risk Assessment" Toxins 12, no. 4: 249. https://doi.org/10.3390/toxins12040249
APA StyleSilva, L. J. G., Teixeira, A. C., Pereira, A. M. P. T., Pena, A., & Lino, C. M. (2020). Ochratoxin A in Beers Marketed in Portugal: Occurrence and Human Risk Assessment. Toxins, 12(4), 249. https://doi.org/10.3390/toxins12040249