Prebiotic Therapy with Inulin Associated with Low Protein Diet in Chronic Kidney Disease Patients: Evaluation of Nutritional, Cardiovascular and Psychocognitive Parameters
Abstract
:1. Introduction
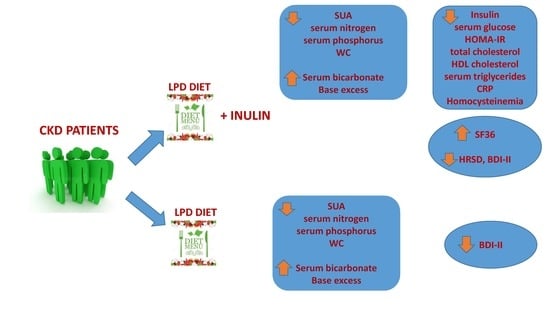
2. Results
2.1. Changes Observed between T0 and T1 in LPD Group
2.2. Changes Observed between T0 and T1 in LPD + Inulin Group
2.3. Changes Observed between T0 and T2 in LPD Group
2.4. Changes Observed between T0 and T2 in LPD + Inulin Group
2.5. Differences in Metabolic and Clinical Parameters between LPD Group and LPD + Inulin Group at T2
2.6. Changes in Psychocognitive Parameters between T0 and T2 in the Two Groups
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Design and Subjects
5.2. Patients
5.2.1. Laboratory Measurements
5.2.2. Anthropometric Assessments
5.2.3. Carotid Intima-Media Thickness Assessment (IMT) and Flow-Mediated Dilation Brachial Artery (FMD)
5.2.4. Renal Resistive Index (RRI)
5.2.5. Psychological and Cognitive Tests
Short Form (SF-36) Health Survey
The Mini-Mental State Examination (MMSE)
The Hamilton Depression Rating Scale (HAM-D)
Beck Depression Inventory-II (BDI-II)
5.3. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esgalhado, M.; Kemp, J.A.; Damasceno, N.R.; Fouque, D.; Mafra, D. Short-chain fatty acids: A link between prebiotics and microbiota in chronic kidney disease. Futur. Microbiol. 2017, 12, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Briskey, D.; Tucker, P.; Johnson, D.W.; Coombes, J.S. The role of the gastrointestinal tract and microbiota on uremic toxins and chronic kidney disease development. Clin. Exp. Nephrol. 2016, 21, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Molfino, A.; Testorio, M.; Perrotta, A.; Currado, A.; Pintus, G.; Pietrucci, D.; Unida, V.; La Rocca, D.; Biocca, S.; et al. Effect of Low-Protein Diet and Inulin on Microbiota and Clinical Parameters in Patients with Chronic Kidney Disease. Nutrients 2019, 11, 3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, W.L.; Savoj, J.; Nakata, M.B.; Vaziri, N.D. Altered microbiome in chronic kidney disease: Systemic effects of gut-derived uremic toxins. Clin. Sci. 2018, 132, 509–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, H.J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, W.L.; Kalantar-Zadeh, K.; Vaziri, N.D. The Gut as a Source of Inflammation in Chronic Kidney Disease. Nephron 2015, 130, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.; Barnes, J.L.; Astroth, K.S. Gastrointestinal Microbiota in Patients with Chronic Kidney Disease: A Systematic Review. Adv. Nutr. 2019, 10, 888–901. [Google Scholar] [CrossRef]
- Katsimichas, T.; Antonopoulos, A.S.; Katsimichas, A.; Ohtani, T.; Sakata, Y.; Tousoulis, D. The intestinal microbiota and cardiovascular disease. Cardiovasc. Res. 2019, 115, 1471–1486. [Google Scholar] [CrossRef]
- Ramezani, A.; Raj, D.S. The Gut Microbiome, Kidney Disease, and Targeted Interventions. J. Am. Soc. Nephrol. 2013, 25, 657–670. [Google Scholar] [CrossRef] [Green Version]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.; Zhao, Y.-Y.; Pahl, M.V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: The nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transplant. 2015, 31, 737–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, H.-P.; Kleessen, B.; Blaut, M. Effects of inulin on faecal bifidobacteria in human subjects. Br. J. Nutr. 1999, 82, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscaritoli, M.; Molfino, A.; Bollea, M.R.; Fanelli, F.R. Malnutrition and wasting in renal disease. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 378–383. [Google Scholar] [CrossRef]
- Rhee, C.M.; Ahmadi, S.-F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachexia Sarcopenia Muscle 2017, 9, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement From the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccio, E.; Di Nuzzi, A.; Pisani, A. Nutritional treatment in chronic kidney disease: The concept of nephroprotection. Clin. Exp. Nephrol. 2014, 19, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Brunori, G.; Di Iorio, B.R.; D’Alessandro, C.; Pasticci, F.; Cosola, C.; Bellizzi, V.; Bolasco, P.; Capitanini, A.; Fantuzzi, A.L.; et al. Nutritional treatment of advanced CKD: Twenty consensus statements. J. Nephrol. 2018, 31, 457–473. [Google Scholar] [CrossRef] [Green Version]
- Kraut, J.A. Effect of metabolic acidosis on progression of chronic kidney disease. Am. J. Physiol. Physiol. 2011, 300, F828–F829. [Google Scholar] [CrossRef] [Green Version]
- Ritter, C.S.; Slatopolsky, E. Phosphate Toxicity in CKD: The Killer among Us. Clin. J. Am. Soc. Nephrol. 2016, 11, 1088–1100. [Google Scholar] [CrossRef] [Green Version]
- Gross, P.; Six, I.; Kamel, S.A.; Massy, Z. Vascular toxicity of phosphate in chronic kidney disease: Beyond vascular calcification. Circ. J. 2014, 78, 2339–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, D.; Recio-Mayoral, A.; Chitalia, N.; Kaski, J.C. Insulin Resistance, Inflammation, and Vascular Disease in Nondiabetic Predialysis Chronic Kidney Disease Patients. Clin. Cardiol. 2011, 34, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Bibbò, S.; Gasbarrini, A.; Cammarota, G. Therapeutic modulation of gut microbiota: Current clinical applications and future perspectives. Curr. Drug Targets 2014, 15, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Cerdó, T.; García-Santos, J.; Bermúdez, M.G.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, N.C.; Groen, A.K.; Romijn, J.A.; Nieuwdorp, M. Gut Microbiota in Obesity and Undernutrition. Adv. Nutr. 2016, 7, 1080–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laviano, A.; Inui, A.; Meguid, M.M.; Molfino, A.; Conte, C.; Fanelli, F.R. NPY and brain monoamines in the pathogenesis of cancer anorexia. Nutrients 2008, 24, 802–805. [Google Scholar] [CrossRef]
- Klein, S.; Allison, D.; Heymsfield, S.B.; Kelley, D.E.; Leibel, R.L.; Nonas, C.; Kahn, R. Waist Circumference and Cardiometabolic Risk: A Consensus Statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity 2007, 15, 1061–1067. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Monti, M.; Muscaritoli, M. Omega-3 Polyunsaturated Fatty Acids in Critical Illness: Anti-Inflammatory, Proresolving, or Both? Oxidative Med. Cell. Longev. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, P.; Alam, S.F. Role of homocysteinee in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Meyrel, M.; Varin, L.; Detaint, B.; Mouaffak, F. The intestinal microbiota: A new player in depression? Encephale 2018, 44, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G. Microbiota-Gut-Brain Axis and Cognitive Function. Adv. Exp. Med. Biol. 2014, 817, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.J.; Swett, K.; Agarwal, S.K.; Folsom, A.R.; Fox, E.R.; Loehr, L.R.; Ni, H.; Rosamond, W.D.; Chang, P.P. Systolic blood pressure levels among adults with hypertension and incident cardiovascular events: The atherosclerosis risk in communities study. JAMA Intern. Med. 2014, 174, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Price, J.F.; Tzoulaki, I.; Lee, A.J.; Fowkes, F.G.R. Ankle brachial index and intima media thickness predict cardiovascular events similarly and increased prediction when combined. J. Clin. Epidemiol. 2007, 60, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Stevens, L.A.; Zhang, Y.; Hendriksen, S.; Kusek, J.W.; Van Lente, F. For the Chronic Kidney Disease Epidemiology Collaboration*; Greene, T. Using Standardized Serum Creatinine Values in the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.Y.; Solomon, S.D. A Clinician’s Guide to Tissue Doppler Imaging. Circulation 2006, 113, 396–398. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Lins, L.; Carvalho, F.M. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Hamilton, M. Development of a Rating Scale for Primary Depressive Illness. Br. J. Soc. Clin. Psychol. 1967, 6, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Steer, R.A.; Rissmiller, D.J.; Beck, A.T. Use of the Beck Depression Inventory-II with depressed geriatric inpatients. Behav. Res. Ther. 2000, 38, 311–318. [Google Scholar] [CrossRef]
| Parameter | LPD + Inulin Group N = 18 | LPD Group N = 23 | p Value |
|---|---|---|---|
| Male | 10 (54%) | 15 (57%) | |
| BMI (kg/m2) | 29.01 ± 3.95 | 28.90 ± 3.46 | 0.07 |
| WC (cm) | 105.0 ± 10.6 | 104.0 ± 9.7 | 0.756 |
| Age (years) | 62.88 ± 7.37 | 60.0 ± 9.9 | 0.264 |
| Serum creatinine (mg/dL) | 2.64 ± 0.72 | 2.27 ± 0.42 | 0.177 |
| eGFR (mL/min) | 24.72 ± 6.92 | 29.61 ± 8.28 | 0.786 |
| Serum nitrogen (mg/dL) | 112.76 ± 29.71 | 113.00 ± 37.14 | 0.982 |
| Serum uric acid (mg/dL) | 6.15 ± 1.01 | 5.92 ± 1.32 | 0.544 |
| Serum glucose (mg/dL) | 97.94 ± 10.07 | 95.15 ± 11.28 | 0.871 |
| Serum phosphorus (mg/dL) | 4.62 ± 0.44 | 4.89 ± 0.65 | 0.139 |
| Serum sodium (mEq/L) | 143.45 ± 4.7 | 142.10 ± 1.97 | 0.219 |
| Total cholesterol (mg/dL) | 201.16 ± 45.03 | 211.55 ± 37.12 | 0.220 |
| HDL cholesterol (mg/dL) | 43.86 ± 7.11 | 46.05 ± 8.42 | 0.376 |
| Serum triglycerides (mg/dL) | 130.11 ± 49.80 | 120.11 ± 38.80 | 0.473 |
| Serum insulin (µU/mL) | 12.34 ± 4.53 | 9.63 ± 5.42 | 0.381 |
| HOMA–IR | 2.95 ± 1.21 | 2.26 ± 1.33 | 0.360 |
| CRP (mg/L) | 5.68 ± 3.63 | 5.23 ± 2.98 | 0.665 |
| BE (mEq/L) | −3.38 ± 3.68 | −3.06 ± 3.28 | 0.307 |
| HCO3− (mEq/L) | 22.41± 3.39 | 21.53 ± 3.38 | 0.381 |
| Serum homocysteine (mg/dL) | 23.87 ± 12.31 | 24.95 ± 14.11 | 0.798 |
| IMT (mm) | 0.95 ± 0.22 | 0.92 ± 0.16 | 0.987 |
| FMD (%) | 9.73 ± 6.32 | 13.99 ± 7.61 | 0.944 |
| RRI | 0.71 ± 0.05 | 0.69 ± 0.09 | 0.403 |
| BDI-II | 7.18 ± 5.84 | 8.05 ± 5.90 | 0.941 |
| HAM-D | 13.62 ± 5.11 | 11.72 ± 5.72 | 0.762 |
| MMSE | 25.58 ± 2.76 | 26.11 ± 2.39 | 0.235 |
| Parameter | T0 | T1 | T2 | p Value * | p Value # |
|---|---|---|---|---|---|
| BE (mmol/L) | −3.06 ± 3.28 | −1.24 ± 2.67 | −1.02 ± 2.45 | 0.045 | 0.021 |
| HCO3−(mEq/L) | 21.53 ± 3.38 | 23.89 ± 2.81 | 24.06 ± 2.56 | 0.013 | 0.006 |
| Serum uric acid (mg/dL) | 5.92 ± 1.32 | 5.16 ± 1.23 | 4.98 ± 1.01 | 0.049 | 0.009 |
| Serum nitrogen (mg/dL) | 113.00 ± 37.14 | 89.39 ± 43.85 | 85.06 ± 34.12 | 0.055 | 0.010 |
| Serum phosphorus (mg/dL) | 4.89 ± 0.65 | 4.58 ± 0.33 | 4.4 ± 0.54 | 0.047 | 0.008 |
| WC (cm) | 104.0 ± 9.7 | 101.2 ± 10.1 | 99.1 ± 6.5 | 0.342 | 0.048 |
| BMI (kg/m2) | 28.90 ± 3.46 | 27.67 ± 3.09 | 27.57 ± 2.45 | 0.210 | 0.055 |
| Parameter | T0 | T1 | T2 | p Value * | p Value # |
|---|---|---|---|---|---|
| BE (mmol/L) | −3.38 ± 3.68 | −0.78 ± 2.03 | −0.68 ± 1.98 | 0.012 | 0.009 |
| HCO3− (mEq/L) | 22.41± 3.39 | 25.04 ± 2.01 | 25.36 ± 3.16 | 0.007 | 0.010 |
| Serum uric acid (mg/dL) | 6.15 ± 1.01 | 5.41 ± 1.13 | 5.33 ± 1.2 | 0.046 | 0.033 |
| Serum nitrogen (mg/dL) | 112.76 ± 29.71 | 85.26 ± 19.88 | 83.23 ± 26.89 | 0.002 | 0.003 |
| Serum phosphorus (mg/dL) | 4.62 ± 0.44 | 4.2 ± 0.55 | 4.12 ± 0.57 | 0.016 | 0.005 |
| Serum sodium (mmol/L) | 143.45 ± 4.7 | 140.23 ± 3.37 | 139.02 ± 3.56 | 0.024 | 0.024 |
| CRP (mg/L) | 5.68 ± 3.63 | 3.84 ± 2.37 | 3.67 ± 1.88 | 0.080 | 0.044 |
| Serum homocysteine (mg/dL) | 23.87 ± 12.31 | 20.33 ± 9.98 | 16.34 ± 9.11 | 0.350 | 0.044 |
| Serum insulin (µU/mL) | 12.34 ± 4.53 | 10.30 ± 5.80 | 8.48 ± 3.72 | 0.247 | 0.008 |
| Serum glucose (mg/dL) | 97.94 ± 10.39 | 91.43 12.30 | 88.94 ± 12.15 | 0.095 | 0.022 |
| HOMA-IR | 2.95 ± 1.21 | 2.72 ± 1.38 | 1.95 ± 0.68 | 0.598 | 0.004 |
| Total cholesterol (mg/dL) | 201.16 ± 45.03 | 185.07 ± 28.67 | 166.22 ± 33.29 | 0.209 | 0.012 |
| HDL cholesterol (mg/dL) | 43.86 ± 7.11 | 46.10 ± 10.69 | 53.00 ± 8.34 | 0.464 | <0.001 |
| Serum triglycerides (mg/dL) | 130.11 ± 49.80 | 125.0 ± 48.83 | 97.41 ± 29.21 | 0.757 | 0.016 |
| WC (cm) | 105.00 ± 10.6 | 101.83 ± 9.58 | 98.83 ± 8.85 | 0.353 | 0.049 |
| BMI (kg/m2) | 29.01 ± 3.95 | 27.80 ± 3.66 | 27.16 ± 2.12 | 0.347 | 0.061 |
| Parameter | LPD + Inulin Group N = 18 | LPD Group N = 23 | p Value |
|---|---|---|---|
| Total cholesterol (mg/dL) | 166.22 ± 33.29 | 217.05 ± 31.9 | <0.001 |
| HDL cholesterol (mg/dL) | 53.00 ± 8.34 | 46.11 ± 9.62 | 0.020 |
| Triglycerides (mg/dL) | 97.41 ± 29.21 | 125.00 ± 50.10 | 0.044 |
| Serum insulin (µU/mL) | 8.48 ± 3.72 | 12.03 ± 4.91 | 0.015 |
| Serum homocysteine (mg/dL) | 16.34 ± 9.11 | 23.07 ± 12.04 | 0.056 |
| RRI | 0.69 ± 0.04 | 0.73 ± 0.08 | 0.060 |
| Parameter | LPD + Inulin Group N = 18 | LPD Group N = 23 | ||||
|---|---|---|---|---|---|---|
| T0 | T2 | p Value | T0 | T2 | p Value | |
| BDI-II | 7.18 ± 5.84 | 3.80 ± 3.30 | 0.028 | 8.05 ± 5.90 | 4.29 ± 5.04 | 0.025 |
| HAM-D | 13.62 ± 5.11 | 7.26 ± 5.93 | <0.001 | 11.72 ± 5.72 | 9.16 ± 4.94 | 0.111 |
| MMSE | 25.58 ± 2.76 | 27.15 ± 2.58 | 0.086 | 26.11 ± 2.39 | 25.88 ± 1.40 | 0.692 |
| SF36 physical functioning | 50.52 ± 27.82 | 72.16 ± 28.68 | 0.028 | 63.95 ± 27.14 | 64.98± 26.92 | 0.909 |
| SF36 bodily pain | 61.86 ± 18.76 | 76.34 ± 20.43 | 0.034 | 64.88 ± 21.60 | 64.44 ± 21.52 | 0.945 |
| SF36 social functioning | 75.16 ± 16.59 | 89.98 ± 14.51 | 0.007 | 76.88 ± 13.23 | 82.33 ± 13.42 | 0.172 |
| SF36 general health perception | 44.33 ± 17.28 | 64.33 ± 16.31 | <0.001 | 45.88 ± 17.34 | 54.27± 17.20 | 0.106 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, S.; Mazzaferro, S.; Muscaritoli, M.; Mastroluca, D.; Testorio, M.; Perrotta, A.; Esposito, Y.; Carta, M.; Campagna, L.; Di Grado, M.; et al. Prebiotic Therapy with Inulin Associated with Low Protein Diet in Chronic Kidney Disease Patients: Evaluation of Nutritional, Cardiovascular and Psychocognitive Parameters. Toxins 2020, 12, 381. https://doi.org/10.3390/toxins12060381
Lai S, Mazzaferro S, Muscaritoli M, Mastroluca D, Testorio M, Perrotta A, Esposito Y, Carta M, Campagna L, Di Grado M, et al. Prebiotic Therapy with Inulin Associated with Low Protein Diet in Chronic Kidney Disease Patients: Evaluation of Nutritional, Cardiovascular and Psychocognitive Parameters. Toxins. 2020; 12(6):381. https://doi.org/10.3390/toxins12060381
Chicago/Turabian StyleLai, Silvia, Sandro Mazzaferro, Maurizio Muscaritoli, Daniela Mastroluca, Massimo Testorio, Adolfo Perrotta, Ylenia Esposito, Maria Carta, Linda Campagna, Marta Di Grado, and et al. 2020. "Prebiotic Therapy with Inulin Associated with Low Protein Diet in Chronic Kidney Disease Patients: Evaluation of Nutritional, Cardiovascular and Psychocognitive Parameters" Toxins 12, no. 6: 381. https://doi.org/10.3390/toxins12060381
APA StyleLai, S., Mazzaferro, S., Muscaritoli, M., Mastroluca, D., Testorio, M., Perrotta, A., Esposito, Y., Carta, M., Campagna, L., Di Grado, M., Ramaccini, C., De Leo, S., Galani, A., Amabile, M. I., & Molfino, A. (2020). Prebiotic Therapy with Inulin Associated with Low Protein Diet in Chronic Kidney Disease Patients: Evaluation of Nutritional, Cardiovascular and Psychocognitive Parameters. Toxins, 12(6), 381. https://doi.org/10.3390/toxins12060381