Biomonitoring of Aflatoxin B1 and Deoxynivalenol in a Rural Pakistan Population Using Ultra-Sensitive LC-MS/MS Method
Abstract
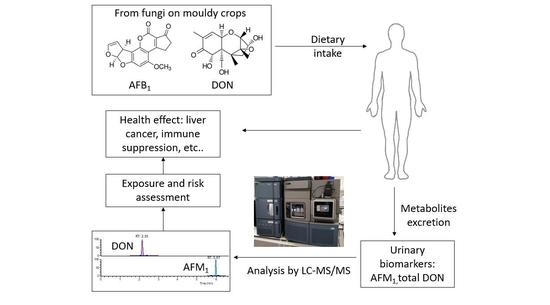
:1. Introduction
2. Results
2.1. Analytical Method Validation
2.2. Demographic Characteristics
2.3. Exposure of Population to AFB1
2.4. Exposure of Population to DON
2.5. Factors that Influence Urinary AFM1/DON Levels
2.6. Association of Urinary AFM1/DON Levels with Food Intake
2.7. Risk Evaluation for AFB1 and DON Exposure
3. Discussion
3.1. Demographic Characteristics and Correlation with Urinary Biomarkers
3.2. Exposure Levels of DON and AFB1
3.3. Correlation between Urinary AFM1 Level and Food Consumption
3.4. Risk Assessment of DON and AFB1
4. Conclusions
5. Materials and Methods
5.1. Study Population and Sampling
5.2. Food AFB 1 and DON Extraction
5.3. Urinary AFB1 Biomarker Extraction
5.4. Urinary DON Biomarker Extraction
5.5. LC-MS/MS Analysis
5.6. Daily Intake Estimation for AFB1 and DON
5.7. Risk Assessment for AFB1 and DON
5.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in food and feed: An overview on prevalence, detection and control strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef] [PubMed]
- Brera, C.; Caputi, R.; Miraglia, M.; Iavicoli, I.; Salerno, A.; Carelli, G. Exposure assessment to mycotoxins in workplaces: Aflatoxins and ochratoxin a occurrence in airborne dusts and human sera. Microchem. J. 2002, 73, 167–173. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Outcome of the public consultation on the draft scientific opinion of the efsa panel on contaminants in the food chain (CONTAM) on acrylamide in food. EFSA Support. Publ. 2015, 12. [Google Scholar] [CrossRef]
- Baan, R.; Grosse, Y.; Straif, K.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part F: Chemical agents and related occupations. Lancet Oncol. 2009, 10, 1143–1144. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Watson, S.; Routledge, M.N. Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Saf. 2016, 4, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Kamala, A.; Shirima, C.; Jani, B.; Bakari, M.; Sillo, H.; Rusibamayila, N.; De Saeger, S.; Kimanya, M.; Gong, Y.; Simba, A.; et al. Outbreak of an acute aflatoxicosis in Tanzania during 2016. World Mycotoxin. J. 2018, 11, 311–320. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Cardwell, K.; Hounsa, A.; Egal, S.; Turner, P.C.; Hall, A.J.; Wild, C.P. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: Cross sectional study. BMJ 2002, 325, 20–21. [Google Scholar] [CrossRef] [Green Version]
- Turner, P.C.; Moore, S.E.; Hall, A.J.; Prentice, A.M.; Wild, C.P. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ. Health Perspect. 2003, 111, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.Y.; Hounsa, A.; Egal, S.; Turner, P.C.; Sutcliffe, A.E.; Hall, A.J.; Cardwell, K.; Wild, C.P. Postweaning exposure to aflatoxin results in impaired child growth: A longitudinal study in benin, West Africa. Environ. Health Perspect. 2004, 112, 1334–1338. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Wilson, S.; Mwatha, J.K.; Routledge, M.N.; Castelino, J.M.; Zhao, B.; Kimani, G.; Kariuki, H.C.; Vennervald, B.J.; Dunne, D.W.; et al. Aflatoxin exposure may contribute to chronic hepatomegaly in Kenyan school children. Environ. Health Perspect. 2012, 120, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Routledge, M.N.; Gong, Y.Y. Molecular epidemiology of aflatoxin exposure and child health. Mutagenesis 2012, 27, 797. [Google Scholar]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef] [PubMed]
- WHO. 72nd Report of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA), in Technical report series. In Evaluation of Certain Contaminants in Food; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Sahar, N.; Ahmed, M.; Parveen, Z.; Ilyas, A.; Bhutto, A. Screening of mycotoxins in wheat, fruits and vegetables grown in Sindh, Pakistan. Pak. J. Bot. 2009, 41, 337–341. [Google Scholar]
- Khatoon, S.; Hanif, N.Q.; Tahira, I.; Sultana, N.; Sultana, K.; Ayub, N. Natural occurrence of aflatoxins, zearalenone and trichothecenes in maize grown in pakistan. Pak. J. Bot. 2012, 44, 231–236. [Google Scholar]
- Majeed, S.; De Boevre, M.; De Saeger, S.; Rauf, W.; Tawab, A.; Habib, F.-E.; Rahman, M.; Iqbal, M. Multiple mycotoxins in rice: Occurrence and health risk assessment in children and adults of punjab, Pakistan. Toxins 2018, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, S.Z.; Usman, S.; Razis, A.F.A.; Ali, N.B.; Saif, T.; Asi, M.R. Assessment of deoxynivalenol in wheat, corn and its products and estimation of dietary intake. Int. J. Environ. Res. Public Health 2020, 17, 5602. [Google Scholar] [CrossRef]
- Turner, P.C.; Rothwell, J.A.; White, K.L.; Gong, Y.Y.; Cade, J.E.; Wild, C.P. Urinary deoxynivalenol is correlated with cereal intake in individuals from the United Kingdom. Environ. Health Perspect. 2007, 116, 21–25. [Google Scholar] [CrossRef]
- Srey, C.; Kimanya, M.E.; Routledge, M.N.; Shirima, C.P.; Gong, Y.Y. Deoxynivalenol exposure assessment in young children in Tanzania. Mol. Nutr. Food Res. 2014, 58, 1574–1580. [Google Scholar] [CrossRef]
- Ashiq, S. Natural occurrence of mycotoxins in food and feed: Pakistan perspective. Compr. Rev. Food Sci. Food Saf. 2014, 14, 159–175. [Google Scholar] [CrossRef]
- Ismail, A.; Gonçalves, B.L.; De Neeff, D.V.; Ponzilacqua, B.; Coppa, C.C.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; De Oliveira, C.A.F. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, H.; Kay, P.; Slack, R.; Gong, Y.Y. Assessment of arsenic species in human hair, toenail and urine and their association with water and staple food. J. Expo. Sci. Environ. Epidemiol. 2018, 29, 624–632. [Google Scholar] [CrossRef] [PubMed]
- European Commision (EC) No.1881/2006. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 15 August 2020).
- Li, Y.; Su, J.J.; Qin, L.L.; Yang, C.; Ban, K.C.; Yan, R.Q. Synergistic effect of hepatitis b virus and aflatoxin b1 in hepatocarcinogenesis in tree shrews. Ann. Acad. Med. Singap. 1999, 28, 67–71. [Google Scholar]
- Kew, M.C. Synergistic interaction between aflatoxin b1 and hepatitis b virus in hepatocarcinogenesis. Liver Int. 2003, 23, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, M.C.; Atanda, O.O.; Ezekiel, C.N. Risk assessment of mycotoxins in stored maize grains con-sumed by infants and young children in Nigeria. Children 2017, 4, 58. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liang, B.; Zhang, Y.; Zhong, X.; Luo, X.; Huang, J.; Wang, Y.; Cheng, W.; Chen, K. Probabilistic risk assessment of dietary exposure to aflatoxin b1 in Guangzhou, China. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, C.J.; Chou, S.R.; Hsieh, L.L.; Wang, L.Y.; Tsai, W.Y.; Ahsan, H.; Santella, R.M. Association of aflatoxin b(1)-albumin adduct levels with hepatitis b surface antigen status among adolescents in Taiwan. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 1223–1226. [Google Scholar]
- Jonsyn-Ellis, F.E. Seasonal variation in exposure frequency and concentration levels of aflatoxins and ochra-toxins in urine samples of boys and girls. Mycopathologia 2001, 152, 35–40. [Google Scholar] [CrossRef]
- Tao, P.; Zhi-Ming, L.; Tang-Wei, L.; Le-Qun, L. Associated factors in modulating aflatoxin B1-albumin adduct level in three Chinese populations. Dig. Dis. Sci. 2005, 50, 525–532. [Google Scholar] [CrossRef]
- Redzwan, S.M.; Rosita, J.; Sokhini, A.M.; Aqilah, A.N.; Wang, J.-S.; Kang, M.-S.; Zuraini, A. Detection of serum afb1-lysine adduct in Malaysia and its association with liver and kidney functions. Int. J. Hyg. Environ. Health 2014, 217, 443–451. [Google Scholar] [CrossRef]
- Van Vleet, T.R.; Mace, K.; Coulombe, R.A. Comparative aflatoxin b-1 activation and cytotoxicity in hu-man bronchial cells expressing cytochromes P450 1A2 and 3A4. Cancer Res. 2002, 62, 105–112. [Google Scholar] [PubMed]
- Ediage, E.N.; Di Mavungu, J.D.; Song, S.; Sioen, I.; De Saeger, S. Multimycotoxin analysis in urines to assess infant exposure: A case study in Cameroon. Environ. Int. 2013, 57, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, N.; Blaszkewicz, M.; Hossain, K.; Degen, G.H. Determination of aflatoxin M 1 in urine samples indicates frequent dietary exposure to aflatoxin B 1 in the Bangladeshi population. Int. J. Hyg. Environ. Health 2017, 220, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Ayelign, A.; Woldegiorgis, A.Z.; Adish, A.; De Boevre, M.; Heyndrickx, E.; De Saeger, S. Assessment of aflatoxin exposure among young children in Ethiopia using urinary bi-omarkers. Food Addit. Contam. Part A Chem. Anal. Control. Exposure Risk Assess. 2017, 34, 606–1616. [Google Scholar]
- Chen, G.; Gong, Y.Y.; Kimanya, M.E.; Shirima, C.P.; Routledge, M.N. Comparison of urinary aflatoxin M1 and aflatoxin albumin adducts as biomarkers for as-sessing aflatoxin exposure in Tanzanian children. Biomarkers 2018, 23, 131–136. [Google Scholar] [CrossRef]
- Deng, C.; Li, C.; Zhou, S.; Wang, X.; Xu, H.; Wang, D.; Gong, Y.Y.; Routledge, M.N.; Zhao, Y.; Wu, Y. Risk assessment of deoxynivalenol in high-risk area of China by human biomonitoring using an improved high throughput UPLC-MS/MS method. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Shephard, G.S.; Burger, H.-M.; Gambacorta, L.; Gong, Y.Y.; Krska, R.; Rheeder, J.P.; Solfrizzo, M.; Srey, C.; Sulyok, M.; Visconti, A.; et al. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food Chem. Toxicol. 2013, 62, 217–225. [Google Scholar] [CrossRef]
- Šarkanj, B.; Ezekiel, C.N.; Turner, P.C.; Abia, W.A.; Rychlik, M.; Krska, R.; Sulyok, M.; Warth, B. Ultra-sensitive, stable isotope assisted quantification of multiple urinary mycotoxin exposure biomarkers. Anal. Chim. Acta 2018, 1019, 84–92. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, T.; Thorgeirsson, S.S.; Zhan, Q.; Chen, J.-G.; Park, J.-H.; Lu, P.; Hsia, C.C.; Wang, N.; Xu, L.; et al. Dramatic reduction of liver cancer incidence in young adults: 28 year follow-up of etiological interventions in an endemic area of China. Carcinogenesis 2013, 34, 1800–1805. [Google Scholar] [CrossRef]
- Jolly, P.; Jiang, Y.; Ellis, W.; Awuah, R.; Nnedu, O.; Phillips, T.; Wang, J.-S.; Afriyie-Gyawu, E.; Tang, L.; Person, S.; et al. Determinants of aflatoxin levels in Ghanaians: Sociodemographic factors, knowledge of aflatoxin and food handling and consumption practices. Int. J. Hyg. Environ. Health 2006, 209, 345–358. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Warth, B.; Ogara, I.M.; Abia, W.A.; Ezekiel, V.C.; Atehnkeng, J.; Sulyok, M.; Turner, P.C.; Tayo, G.O.; Krska, R.; et al. Mycotoxin exposure in rural residents in northern Nigeria: A pilot study using multi-urinary biomarkers. Environ. Int. 2014, 66, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Hossain, K.; Blaszkewicz, M.; Rahman, M.; Mohanto, N.C.; Alim, A.; Degen, G.H. Occurrence of aflatoxin M1 in urines from rural and urban adult cohorts in Bangladesh. Arch. Toxicol. 2015, 90, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, Q.Z.M.A.; Rasul, G.; Afzaal, M. Climate Change Indicators of Pakistan; Pakistan Meteorological Department: Islamabad, Pakistan, 2009.
- Magan, N.; Medina, A.; Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
- Nesic, K. Mycotoxins—Climate impact and steps to prevention based on prediction. Acta Veterinaria-Beograd 2018, 68, 1–15. [Google Scholar]
- Romero, A.D.C.; Ferreira, T.R.B.; Dias, C.T.S.; Calori-Domingues, M.A.; Da Glória, E.M. Occurrence of AFM1 in urine samples of a Brazilian population and association with food consumption. Food Control. 2010, 21, 554–558. [Google Scholar] [CrossRef]
- Akhtar, S.; Riaz, M.; Naeem, I.; Gong, Y.Y.; Ismail, A.; Hussain, M.; Akram, K. Risk assessment of aflatoxins and selected heavy metals through intake of branded and non-branded spices collected from the markets of Multan city of Pakistan. Food Control. 2020, 112, 107132. [Google Scholar] [CrossRef]
- Ismail, A.; Akhtar, S.; Riaz, M.; Gong, Y.Y.; Routledge, M.N.; Naeem, I. Prevalence and exposure assessment of aflatoxins through black tea consumption in the Multan City of Pakistan and the impact of tea making process on aflatoxins. Front. Microbiol. 2020, 11, 446–454. [Google Scholar] [CrossRef]
- Yunus, A.W.; Ullah, A.; Lindahl, J.F.; Anwar, Z.; Ullah, A.; Saif, S.; Ali, M.; Bin Zahur, A.; Irshad, H.; Javaid, S.; et al. Aflatoxin contamination of milk produced in peri-urban farms of pakistan: Prevalence and contributory factors. Front. Microbiol. 2020, 11, 159. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, H.; Slack, R.J.; Kay, P.; Gong, Y.Y. Refinement of arsenic attributable health risks in rural Pakistan using population specific dietary intake values. Environ. Int. 2017, 99, 331–342. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the scientific committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both Genotoxic and Carcinogenic. EFSA J. 2005, 3, 282. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-C.; Wang, Q.; Yang, H.-I.; Ahsan, H.; Tsai, W.-Y.; Wang, L.-Y.; Chen, S.-Y.; Chen, C.-J.; Santella, R.M. Aflatoxin B1 exposure, hepatitis B virus infection, and hepatocellular carcinoma in Taiwan. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 846–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatti, A.B.H.; Dar, F.S.; Waheed, A.; Shafique, K.; Sultan, F.; Shah, N.H. Hepatocellular carcinoma in Pakistan: National trends and global perspective. Gastroenterol. Res. Pract. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palliyaguru, D.L.; Wu, F. Global geographical overlap of aflatoxin and hepatitis C: Controlling risk factors for liver cancer worldwide. Food Addit. Contam. Part A 2013, 30, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A.; Ashfaq, U.A. Epidemiology of hepatitis C infection in Pakistan: Current estimate and major risk factors. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 63–77. [Google Scholar] [CrossRef]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.L.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2018. Food Chem. Toxicol. 2019, 129, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, 211. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Zhang, L.S.; Hu, X.; Xiao, Y.; Chen, J.S.; Xu, Y.C.; Fremy, J.; Chu, F.S. Correlation of dietary aflatoxin B1 levels with excretion of aflatoxin M1 in human urine. Cancer Res. 1987, 47, 1848–1852. [Google Scholar]
- Wang, J.S.; Qian, G.S.; Zarba, A.; He, X.; Zhu, Y.R.; Zhang, B.C.; Jacobson, L.; Gange, S.J.; Muñoz, A.; Kensler, T.W. Temporal patterns of aflatoxin-albumin adducts in hepatitis B surface antigen-positive and antigen-negative residents of Daxin, Qidong county, people’s republic of China. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 253–261. [Google Scholar]
- Wu, H.C.; Wang, Q.; Yang, H.-I.; Ahsan, H.; Tsai, W.-Y.; Wang, L.-Y.; Chen, S.-Y.; Chen, C.-Y.; Santella, R.M. Urinary 15-F2t-isoprostane, aflatoxin B1 exposure and hepatitis B virus infection and hepato-cellular carcinoma in Taiwan. Carcinogenesis 2008, 29, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Donahue, R.M.; Qureshi, H.; Vermund, S.H. Hepatitis B and hepatitis C in Pakistan: Prevalence and risk factors. Int. J. Infect. Dis. 2009, 13, 9–19. [Google Scholar] [CrossRef] [Green Version]




| AFM1 | DON | ||||
|---|---|---|---|---|---|
| Spiked Level (ng/mL) | Recovery Mean ± SD (%) | CV (%) | Spiked Level (ng/mL) | Recovery Mean ± SD (%) | CV (%) |
| 0.15 | 116 ± 18.5 | 16 | 8 | 91 ± 11.7 | 13 |
| 1 | 98 ± 10.4 | 11 | 25 | 98 ± 8.6 | 9 |
| 15 | 85 ± 11.9 | 14 | 125 | 103 ± 9.5 | 9 |
| Characteristics | Chak-46 | Chak-48 | Chak-49 | BB | BP | KA | All Villages |
|---|---|---|---|---|---|---|---|
| Total | 99 | 13 | 35 | 35 | 24 | 58 | 264 |
| Male , (%) | 63 (64) | 13 (100) | 29 (83) | 11 (31) | 15 (63) | 22 (38) | 153 (58) |
| Age | |||||||
| Mean ± SD (range) | 32.1 ± 17.7 (4–75) | 42.5 ± 12.1 (17–61) | 41.4 ± 17.3 (6–70) | 36.1 ± 17.0(9–65) | 37.6 ± 16.5 (15–65) | 32.9 ± 16.7(9–80) | 35.0 ± 17.5 (4–80) |
| Occupation n (%) | |||||||
| Farmer | 58 (59) | 11 (85) | 29 (83) | 25 (71) | 21 (88) | 39 (67) | 185 (70) |
| Student | 27 (27) | 1 (7.5) | 5 (14) | 6 (17) | 3 (12) | 14 (24) | 56 (21) |
| Other | 14 (14) | 1 (7.5) | 1 (3) | 4 (11) | 0 (0) | 4 (9) | 24 (9) |
| Chapatti * consumption (g/kg b.w./day) | |||||||
| Mean ± SD | 6.4 ± 2.3 (0–12.1) | 7.0 ± 2.1 (4.8–12.6) | 7.2 ± 2.6 (3.0–15.0) | 7.8 ± 2.1 (0–12.1) | 6.9 ± 1.4 (4.4–10.0) | 7.3 ± 2.0 (3.2–13.4) | 7.0 + 2.2 (0–15.0) |
| Rice consumption (g/kg b.w./day) | |||||||
| Consumption rate (%) | 50.5 | 46.2 | 37.1 | 34.3 | 12.5 | 44.8 | 41.7 |
| Mean ± SD (range) | 9.6 ± 3.6 (4.3–19.8) | 7.4 ± 1.6 (4.3–8.8) | 9.1 ± 4.0 (3.9–20.6) | 8.8 ± 2.7 (5.5–12.5) | 8.7 ± 1.1 (7.5–9.6) | 8.8 ± 2.2 (4.5–13.5) | 9.1 ± 3.1 (3.9–20.6) |
| Villages | Chak-46 | Chak-48 | Chak-49 | BB | BP | KA | All Villages |
|---|---|---|---|---|---|---|---|
| Rice * (p = 0.04) | |||||||
| Positive n a (%) | 3/8 (38) | 0/1 (0) | 0/5(0) | 13/17 (76) | 9/15 (60) | 16/16 (100) | 41/62 (66) |
| Mean ± SD (µg/kg) | 5.65 ± 9.51 | nd | nd | 10.17 ± 18.02 | 5.11 ± 11.94 | 1.09 ± 1.40 | 5.04 ± 11.94 |
| Median (range) (µg/kg) | 0.03 (nd–23.58) | nd (nd) | nd (nd) | 2.46 (nd–71.56) | 0.37 (nd–36.88) | 0.42 (0.2–5.26) | 0.38 (nd–71.56) |
| Wheat (p = 0.93) | |||||||
| Positive n (%) | 1/40 (3) | 1/45 (2) | 1/51 (2) | 0/13 (0) | 0/17 (0) | 0/29 (0) | 3/195 (2) |
| Mean ± SD (µg/kg) | 0.04 ± 0.03 | 0.03 ± 0.06 | 0.06 ± 0.22 | nd | nd | nd | 0.04 ± 0.12 |
| Median (range) (µg/kg) | nd (nd–0.23) | nd (nd–0.42) | nd (nd–1.59) | nd (nd) | nd (nd) | nd (nd) | nd (nd–1.59) |
| Villages | Chak-46 | Chak-48 | Chak-49 | BB | BP | KA | All Villages |
|---|---|---|---|---|---|---|---|
| DON (p = 0.46) | |||||||
| Positive n (%) | 18/99 (18) | 0/13 (0) | 8/35 (23) | 11/35 (31) | 7/24 (29) | 10/58 (17) | 54/264 (20) |
| Mean ± SD (ng/mL) | 0.166 ± 0.113 | nd | 0.156 ± 0.064 | 0.202 ± 0.187 | 0.169 ± 0.080 | 0.174 ± 0.165 | 0.170 ± 0.129 |
| Median (range) (ng/mL) | nd (nd–0.901) | nd (nd) | nd (nd–0.348) | nd (nd–1.177) | nd (nd–0.388) | nd (nd–1.247) | nd (nd–1.247) |
| AFM1 *** (p < 0.001) | |||||||
| Positive n (%) | 86/99 (87) | 7/13 (54) | 21/35 (60) | 19/35 (54) | 20/24 (83) | 29/58 (50) | 182/264 (69) |
| Mean ± SD (ng/mL) | 0.039 ± 0.015 | 0.022 ± 0.059 | 0.011 ± 0.021 | 0.003 ± 0.004 | 0.037 ± 0.045 | 0.009 ± 0.021 | 0.023 ± 0.048 |
| Median (range) (ng/mL) | 0.015 (nd–0.393) | 0.003 (nd–0.215) | 0.003 (nd–0.114) | 0.002 (nd–0.017) | 0.020 (nd–0.162) | 0.001 (nd–0.137) | 0.004 (nd–0.393) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, L.; Routledge, M.N.; Rasheed, H.; Ismail, A.; Dong, Y.; Jiang, T.; Gong, Y.Y. Biomonitoring of Aflatoxin B1 and Deoxynivalenol in a Rural Pakistan Population Using Ultra-Sensitive LC-MS/MS Method. Toxins 2020, 12, 591. https://doi.org/10.3390/toxins12090591
Xia L, Routledge MN, Rasheed H, Ismail A, Dong Y, Jiang T, Gong YY. Biomonitoring of Aflatoxin B1 and Deoxynivalenol in a Rural Pakistan Population Using Ultra-Sensitive LC-MS/MS Method. Toxins. 2020; 12(9):591. https://doi.org/10.3390/toxins12090591
Chicago/Turabian StyleXia, Lei, Michael N. Routledge, Hifza Rasheed, Amir Ismail, Yao Dong, Tao Jiang, and Yun Yun Gong. 2020. "Biomonitoring of Aflatoxin B1 and Deoxynivalenol in a Rural Pakistan Population Using Ultra-Sensitive LC-MS/MS Method" Toxins 12, no. 9: 591. https://doi.org/10.3390/toxins12090591
APA StyleXia, L., Routledge, M. N., Rasheed, H., Ismail, A., Dong, Y., Jiang, T., & Gong, Y. Y. (2020). Biomonitoring of Aflatoxin B1 and Deoxynivalenol in a Rural Pakistan Population Using Ultra-Sensitive LC-MS/MS Method. Toxins, 12(9), 591. https://doi.org/10.3390/toxins12090591