Extracellular Vesicles and Their Relationship with the Heart–Kidney Axis, Uremia and Peritoneal Dialysis
Abstract
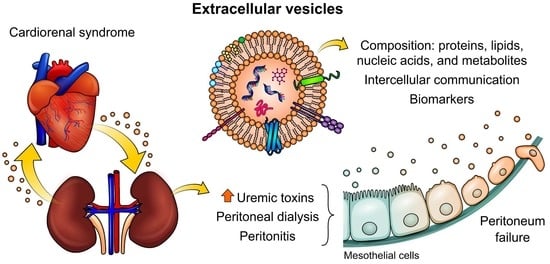
:1. Introduction
2. Cardiorenal Syndrome: Role of Uremic Toxins (UTs) in Peritoneal Dialysis
3. The Importance of Extracellular Vesicles in Heart/Kidney Axis and Peritoneal Dialysis
4. Isolation and Characterization of Extracellular Vesicles (EVs)
4.1. Isolating EVs
4.2. Characterizing EVs
4.2.1. Electron Microscopy
4.2.2. Nanoparticle Tracking Analysis
4.2.3. Dynamic Light Scattering
4.2.4. Flow Cytometry
4.2.5. Proteomic, Transcriptome, Lipidomic and Metabolomic Approaches
4.2.6. Fourier Transform Infrared (FTIR) and Raman Spectroscopies as Diagnostic Tools
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kumar, U.; Wettersten, N.; Garimella, P.S. Cardiorenal Syndrome: Pathophysiology. Cardiol. Clin. 2019, 37, 251–265. [Google Scholar] [CrossRef]
- Barreto, F.C.; Stinghen, A.E.M.; de Oliveira, R.B.; Franco, A.T.B.; Moreno, A.N.; Barreto, D.V.; Pecoits-Filho, R.; Drüeke, T.B.; Massy, Z.A. The quest for a better understanding of chronic kidney disease complications: An update on uremic toxins. J. Bras. Nefrol. 2014, 36, 221–235. [Google Scholar] [CrossRef]
- Falconi, C.A.; da Cruz Junho, C.V.; Fogaça-Ruiz, F.; Vernier, I.C.S.; da Cunha, R.S.; Stinghen, A.E.M.; Carneiro-Ramos, M.S. Uremic Toxins: An Alarming Danger Concerning the Cardiovascular System. Front. Physiol. 2021, 12, 667. [Google Scholar] [CrossRef] [PubMed]
- Favretto, G.; da Cunha, R.S.; Flores Santos, A.; Leitolis, A.; Schiefer, E.M.; Gregório, P.C.; Franco, C.R.C.; Massy, Z.; Dalboni, M.A.; Stinghen, A.E.M. Uremic endothelial-derived extracellular vesicles: Mechanisms of formation and their role in cell adhesion, cell migration, inflammation, and oxidative stress. Toxicol. Lett. 2021, 347, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.I.; De Loor, H.; Bammens, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. p-cresyl sulfate and indoxyl sulfate in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1932–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasian, N.; Goodall, A.H.; Burton, J.O.; Bursnall, D.; Bevington, A.; Brunskill, N.J. Hyperphosphatemia Drives Procoagulant Microvesicle Generation in the Rat Partial Nephrectomy Model of CKD. J. Clin. Med. 2020, 9, 3534. [Google Scholar] [CrossRef] [PubMed]
- Soriano, S.; Carmona, A.; Triviño, F.; Rodriguez, M.; Alvarez-Benito, M.; Martín-Malo, A.; Alvarez-Lara, M.A.; Ramírez, R.; Aljama, P.; Carracedo, J. Endothelial damage and vascular calcification in patients with chronic kidney disease. Am. J. Physiol.-Ren. Physiol. 2014, 307, 1302–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faure, V.; Dou, L.; Sabatier, F.; Cerini, C.; Sampol, J.; Berland, Y.; Brunet, P.; Ddignat-George, F. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J. Thromb. Haemost. 2006, 4, 566–573. [Google Scholar] [CrossRef]
- Mavrakanas, T.A.; Khattak, A.; Singh, K.; Charytan, D.M. Epidemiology and natural history of the cardiorenal syndromes in a cohort with echocardiography. Clin. J. Am. Soc. Nephrol. 2017, 12, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellasi, A.; Di Lullo, L. Cardiorenal Syndrome: An Overview. Adv. Chronic Kidney Dis. 2018, 25, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Ramchandra, R.; Xing, D.T.; Matear, M.; Lambert, G.; Allen, A.M.; May, C.N. Neurohumoral interactions contributing to renal vasoconstriction and decreased renal blood flow in heart failure. Am. J. Physiol. Integr. Comp. Physiol. 2019, 317, R386–R396. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, L.; Bellasi, A.; Barbera, V.; Russo, D.; Russo, L.; Di Iorio, B.; Cozzolino, M.; Ronco, C. Pathophysiology of the cardio-renal syndromes types 1–5: An uptodate. Indian Heart J. 2017, 69, 255–265. [Google Scholar] [CrossRef]
- Liang, K.V.; Williams, A.W.; Greene, E.L.; Redfield, M.M. Acute decompensated heart failure and the cardiorenal syndrome. Crit. Care Med. 2008, 36, S75–S88. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Di Lullo, L. Cardiorenal syndrome. Heart Fail. Clin. 2014, 10, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.N.; Schmidt-Ott, K.M.; Vescovo, G.; House, A.A.; Kellum, J.A.; Ronco, C.; McCullough, P.A. Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: Workgroup statements from the eleventh consensus conference of the acute dialysis quality initiative (ADQI). In Contributions to Nephrology; Karger: Basel, Switzerland, 2013; Volume 182, pp. 117–136. ISBN 9783318024067. [Google Scholar]
- Bagshaw, S.M.; Hoste, E.A.; Braam, B.; Briguori, C.; Kellum, J.A.; McCullough, P.A.; Ronco, C. Cardiorenal syndrome type 3: Pathophysiologic and epidemiologic considerations. In Contributions to Nephrology; Karger: Basel, Switzerland, 2013; Volume 182, pp. 137–157. ISBN 9783318024067. [Google Scholar]
- Di Lullo, L.; House, A.; Gorini, A.; Santoboni, A.; Russo, D.; Ronco, C. Chronic kidney disease and cardiovascular complications. Heart Fail. Rev. 2015, 20, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Goto, S.; Fukagawa, M. Role of uremic toxins for kidney, cardiovascular, and bone dysfunction. Toxins 2018, 10, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-García, J.A.; Fernández-Velasco, M.; Delgado, C.; Delgado, J.F.; Kuro-o, M.; Ruilope, L.M.; Ruiz-Hurtado, G. PTH, vitamin D, and the FGF-23–klotho axis and heart: Going beyond the confines of nephrology. Eur. J. Clin. Investig. 2018, 48, e12902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unver, S.; Kavlak, E.; Gümüsel, H.K.; Celikbilek, F.; Esertas, K.; Muftuoglu, T.; Kirilmaz, A. Correlation between hypervolemia, left ventricular hypertrophy and fibroblast growth factor 23 in hemodialysis patients. Ren. Fail. 2015, 37, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Curione, M.; Amato, S.; Di Bona, S.; Petramala, L.; Cotesta, D.; Letizia, C. Parathyroidectomy erase increased myocardial electrical vulnerability in patients with primary hyperparathyroidism. Int. J. Cardiol. 2010, 141, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Covic, A.; Vervloet, M.; Massy, Z.A.; Torres, P.U.; Goldsmith, D.; Brandenburg, V.; Mazzaferro, S.; Evenepoel, P.; Bover, J.; Apetrii, M.; et al. Bone and mineral disorders in chronic kidney disease: Implications for cardiovascular health and ageing in the general population. Lancet Diabetes Endocrinol. 2018, 6, 319–331. [Google Scholar] [CrossRef]
- Seeherunvong, W.; Abitbol, C.L.; Chandar, J.; Rusconi, P.; Zilleruelo, G.E.; Freundlich, M. Fibroblast growth factor 23 and left ventricular hypertrophy in children on dialysis. Pediatr. Nephrol. 2012, 27, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Su, X.; Huang, W.; Zhang, J.; Peng, C.; Huang, H.; Wu, X.; Huang, H.; Xia, M.; Ling, W. Role of S-adenosylhomocysteine in cardiovascular disease and its potential epigenetic mechanism. Int. J. Biochem. Cell Biol. 2015, 67, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Apetrii, M.; Enache, S.; Siriopol, D.; Burlacu, A.; Kanbay, A.; Kanbay, M.; Scripcariu, D.; Covic, A. A brand-new cardiorenal syndrome in the COVID-19 setting. Clin. Kidney J. 2020, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Nair, N.; Chakraborty, R.; Nemer, L.; Dasgupta, R.; Varian, K. An Update on the Pathophysiology and Treatment of Cardiorenal Syndrome. Cardiol. Res. 2020, 11, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Glassock, R.J. Uremic Toxins: What Are They? An Integrated Overview of Pathobiology and Classification. J. Ren. Nutr. 2008, 18, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Stinghen, A.E.M.; Gonçalves, S.M.; Martines, E.G.; Nakao, L.S.; Riella, M.C.; Aita, C.A.; Pecoits-Filho, R. Increased Plasma and Endothelial Cell Expression of Chemokines and Adhesion Molecules in Chronic Kidney Disease. Nephron Clin. Pract. 2009, 111, c117–c126. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, K.; Malhotra, K.; Sowers, J.; Aroor, A. Uric acid-key ingredient in the recipe for cardiorenal metabolic syndrome. Cardiorenal Med. 2013, 3, 208–220. [Google Scholar] [CrossRef] [Green Version]
- Tamariz, L.; Hernandez, F.; Bush, A.; Palacio, A.; Hare, J.M. Association between serum uric acid and atrial fibrillation: A systematic review and meta-analysis. Heart Rhythm 2014, 11, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Gona, P.; Benjamin, E.J.; Wang, T.J.; Aragam, J.; D’Agostino Sr, R.B.; Kannel, W.B.; Vasan, R.S. Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur. J. Heart Fail. 2010, 12, 812. [Google Scholar] [CrossRef] [Green Version]
- Cancela, A.L.; Santos, R.D.; Titan, S.M.; Goldenstein, P.T.; Rochitte, C.E.; Lemos, P.A.; dos Reis, L.M.; Graciolli, F.G.; Jorgetti, V.; Moysés, R.M. Phosphorus Is Associated with Coronary Artery Disease in Patients with Preserved Renal Function. PLoS ONE 2012, 7, e36883. [Google Scholar] [CrossRef]
- Kestenbaum, B.; Sampson, J.N.; Rudser, K.D.; Patterson, D.J.; Seliger, S.L.; Young, B.; Sherrard, D.J.; Andress, D.L. Serum phosphate levels and mortality risk among people with chronic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 520–528. [Google Scholar] [CrossRef] [Green Version]
- Sage, A.P.; Lu, J.; Tintut, Y.; Demer, L.L. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2011, 79, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, K.R.; Graciolli, F.G.; Dos Reis, L.M.; Pasqualucci, C.A.; Moysés, R.M.A.; Jorgetti, V. Adverse effects of hyperphosphatemia on myocardial hypertrophy, renal function, and bone in rats with renal failure. Kidney Int. 2004, 66, 2237–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmonston, D.; Wolf, M. FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat. Rev. Nephrol. 2020, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.-C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-García, J.A.; Delgado, C.; Fernández-Velasco, M.; Val-Blasco, A.; Rodríguez-Sánchez, E.; Aceves-Ripoll, J.; Gómez-Hurtado, N.; Bada-Bosch, T.; Mérida-Herrero, E.; Hernández, E.; et al. Fibroblast growth factor-23 promotes rhythm alterations and contractile dysfunction in adult ventricular cardiomyocytes. Nephrol. Dial. Transplant. 2019, 34, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Kuczera, P.; Adamczak, M.; Wiecek, A. Fibroblast growth factor-23—A potential uremic toxin. Toxins 2016, 8, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storino, G.F.; Moraes, C.; Saldanha, J.; Mafra, D. Cardiovascular Mortality in Chronic Kidney Patients: The Role of Uremic Toxins. Int. J. Cardiovasc. Sci. 2015, 28, 10–5935. [Google Scholar] [CrossRef]
- Lekawanvijit, S.; Adrahtas, A.; Kelly, D.J.; Kompa, A.R.; Wang, B.H.; Krum, H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur. Heart J. 2010, 31, 1771–1779. [Google Scholar] [CrossRef] [Green Version]
- Ichii, O.; Otsuka-Kanazawa, S.; Nakamura, T.; Ueno, M.; Kon, Y.; Chen, W.; Rosenberg, A.Z.; Kopp, J.B. Podocyte Injury Caused by Indoxyl Sulfate, a Uremic Toxin and Aryl-Hydrocarbon Receptor Ligand. PLoS ONE 2014, 9, e108448. [Google Scholar] [CrossRef] [Green Version]
- Lekawanvijit, S.; Kompa, A.R.; Manabe, M.; Wang, B.H.; Langham, R.G.; Nishijima, F.; Kelly, D.J.; Krum, H. Chronic Kidney Disease-Induced Cardiac Fibrosis Is Ameliorated by Reducing Circulating Levels of a Non-Dialysable Uremic Toxin, Indoxyl Sulfate. PLoS ONE 2012, 7, e41281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yisireyili, M.; Shimizu, H.; Saito, S.; Enomoto, A.; Nishijima, F.; Niwa, T. Indoxyl sulfate promotes cardiac fibrosis with enhanced oxidative stress in hypertensive rats. Life Sci. 2013, 92, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Bergé-Lefranc, D.; Chaspoul, F.; Calaf, R.; Charpiot, P.; Brunet, P.; Gallice, P. Binding of p-Cresylsulfate and p-Cresol to Human Serum Albumin Studied by Microcalorimetry. J. Phys. Chem. B 2010, 114, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Nomura, T.; Sugiyama, S.; Miyazaki, T.; Tsukushi, S.; Tsutsui, S. The protein metabolite hypothesis, a model for the progression of renal failure: An oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int. Suppl. 1997, 62, S23–S28. [Google Scholar]
- Adijiang, A.; Goto, S.; Uramoto, S.; Nishijima, F.; Niwa, T. Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol. Dial. Transplant. 2008, 23, 1892–1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumur, Z.; Niwa, T. Indoxyl Sulfate Inhibits Nitric Oxide Production and Cell Viability by Inducing Oxidative Stress in Vascular Endothelial Cells. Am. J. Nephrol. 2009, 29, 551–557. [Google Scholar] [CrossRef]
- Tumur, Z.; Shimizu, H.; Enomoto, A.; Miyazaki, H.; Niwa, T. Indoxyl Sulfate Upregulates Expression of ICAM-1 and MCP-1 by Oxidative Stress-Induced NF-ĸB Activation. Am. J. Nephrol. 2010, 31, 435–441. [Google Scholar] [CrossRef]
- Yu, M.; Kim, Y.J.; Kang, D.-H. Indoxyl Sulfate–Induced Endothelial Dysfunction in Patients with Chronic Kidney Disease via an Induction of Oxidative Stress. Clin. J. Am. Soc. Nephrol. 2011, 6, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, T.; Katsuki, S.; Chen, M.; Decano, J.L.; Halu, A.; Lee, L.H.; Pestana, D.V.S.; Kum, A.S.T.; Kuromoto, R.K.; Golden, W.S.; et al. Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation Via the Interplay of OATP2B1 and Dll4-Notch Signaling. Circulation 2019, 139, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Yoo, T.-H.; Cho, J.-Y.; Kim, H.C.; Lee, W.-W. Indoxyl sulfate–induced TNF-α is regulated by crosstalk between the aryl hydrocarbon receptor, NF-κB, and SOCS2 in human macrophages. FASEB J. 2019, 33, 10844–10858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization WHO—The Top 10 Causes of Death. Available online: http://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 28 July 2021).
- Andreoli, M.C.C.; Totoli, C. Peritoneal dialysis. Rev. Assoc. Med. Bras. 2020, 66, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreras-Planella, L.; Soler-Majoral, J.; Rubio-Esteve, C.; Lozano-Ramos, S.I.; Franquesa, M.; Bonet, J.; Troya-Saborido, M.I.; Borràs, F.E. Characterization and proteomic profile of extracellular vesicles from peritoneal dialysis efflux. PLoS ONE 2017, 12, e0176987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fülöp, T.; Zsom, L.; Tapolyai, M.B.; Molnar, M.Z.; Abdul Salim, S.; Arany, I.; Hamrahian, M.; Rosivall, L. Peritoneal dialysis: The unique features by compartmental delivery of renal replacement therapy. Med. Hypotheses 2017, 108, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Hamada, C.; Nakayama, M.; Miyazaki, M.; Sherif, A.M.; Harada, T.; Hirano, H. Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: A quantitative study of peritoneal membrane morphology. Clin. J. Am. Soc. Nephrol. 2008, 3, 720–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-Y.; Lin, C.-Y.; Chien, C.-C.; Kan, W.-C.; Tian, Y.-F.; Liao, P.-C.; Wu, H.-Y.; Su, S.-B. Impact of uremic environment on peritoneum: A proteomic view. J. Proteom. 2012, 75, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Lara-Pezzi, E.; Selgas, R.; Ramírez-Huesca, M.; Domínguez-Jiménez, C.; Jiménez-Heffernan, J.A.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; Álvarez, V.; et al. Peritoneal Dialysis and Epithelial-to-Mesenchymal Transition of Mesothelial Cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Aroeira, L.S.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; Del Peso, G.; Jiménez-Heffernan, J.A.; Selgas, R.; López-Cabrera, M. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: Pathologic significance and potential therapeutic interventions. J. Am. Soc. Nephrol. 2007, 18, 2004–2013. [Google Scholar] [CrossRef] [Green Version]
- Karl, Z.J.T.; Khanna, O.N.R.; Leonor, B.F.P.; Ryan, P.; Moore, H.L.; Nielsen, M.P. Peritoneal Equilibration Test. Perit. Dial. Int. 1987, 7, 138–148. [Google Scholar] [CrossRef]
- Carreras-Planella, L.; Soler-Majoral, J.; Rubio-Esteve, C.; Morón-Font, M.; Franquesa, M.; Bonal, J.; Troya-Saborido, M.I.; Borràs, F.E. Proteomic profiling of peritoneal dialysis effluent-derived extracellular vesicles: A longitudinal study. J. Nephrol. 2019, 32, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Corciulo, S.; Nicoletti, M.C.; Mastrofrancesco, L.; Milano, S.; Mastrodonato, M.; Carmosino, M.; Gerbino, A.; Corciulo, R.; Russo, R.; Svelto, M.; et al. AQP1-Containing Exosomes in Peritoneal Dialysis Effluent As Biomarker of Dialysis Efficiency. Cells 2019, 8, 330. [Google Scholar] [CrossRef] [Green Version]
- Bright, R. Cases and Observations, Illustrative of Renal Disease, Accompanied with the Secretion of Albuminous Urine. Br. Foreign Med. Rev. 1839, 8, 121–158. [Google Scholar]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2021, 11, 1757. [Google Scholar] [CrossRef]
- Burger, D.; Schock, S.; Thompson, C.S.; Montezano, A.C.; Hakim, A.M.; Touyz, R.M. Microparticles: Biomarkers and beyond. Clin. Sci. 2013, 124, 423–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, S.; Abou-Arkoub, R.; Sun, S.; Hiremath, S.; Reunov, A.; McCormick, B.B.; Ruzicka, M.; Burger, D. Microparticle formation in peritoneal dialysis: A proof of concept study. Can. J. Kidney Health Dis. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Ridger, V.C.; Boulanger, C.M.; Angelillo-Scherrer, A.; Badimon, L.; Blanc-Brude, O.; Bochaton-Piallat, M.L.; Boilard, E.; Buzas, E.I.; Caporali, A.; Dignat-George, F.; et al. Microvesicles in vascular homeostasis and diseases position paper of the european society of cardiology (ESC) working group on atherosclerosis and vascular biology. Thromb. Haemost. 2017, 117, 1296–1316. [Google Scholar] [CrossRef]
- Pironti, G.; Strachan, R.T.; Abraham, D.; Mon-Wei Yu, S.; Chen, M.; Chen, W.; Hanada, K.; Mao, L.; Watson, L.J.; Rockman, H.A. Circulating Exosomes Induced by Cardiac Pressure Overload Contain Functional Angiotensin II Type 1 Receptors. Circulation 2015, 131, 2120–2130. [Google Scholar] [CrossRef]
- Vasina, E.; Heemskerk, J.W.M.; Weber, C.; Koenen, R.R. Platelets and platelet-derived microparticles in vascular inflammatory disease. Inflamm. Allergy Drug Targets 2010, 9, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Vajen, T.; Mause, S.F.; Koenen, R.R. Microvesicles from platelets: Novel drivers of vascular inflammation. Thromb. Haemost. 2015, 114, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Burbano, C.; Villar-Vesga, J.; Orejuela, J.; Muñoz, C.; Vanegas, A.; Vásquez, G.; Rojas, M.; Castaño, D. Potential Involvement of Platelet-Derived Microparticles and Microparticles Forming Immune Complexes during Monocyte Activation in Patients with Systemic Lupus Erythematosus. Front. Immunol. 2018, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Mobarrez, F.; Svenungsson, E.; Pisetsky, D.S. Microparticles as autoantigens in systemic lupus erythematosus. Eur. J. Clin. Investig. 2018, 48, e13010. [Google Scholar] [CrossRef] [PubMed]
- Ullal, A.J.; Reich, C.F.; Clowse, M.; Criscione-Schreiber, L.G.; Tochacek, M.; Monestier, M.; Pisetsky, D.S. Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. J. Autoimmun. 2011, 36, 173–180. [Google Scholar] [CrossRef]
- Crow, J.; Atay, S.; Banskota, S.; Artale, B.; Schmitt, S.; Godwin, A.K. Exosomes as mediators of platinum resistance in ovarian cancer. Oncotarget 2017, 8, 11917–11936. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.Y.; You, S.; Jung, J.H.; Lee, J.C.; Rho, J.K.; Lee, K.Y.; Freeman, M.R.; Kim, K.P.; Kim, J. Extracellular vesicles shed from gefitinib-resistant nonsmall cell lung cancer regulate the tumor microenvironment. Proteomics 2014, 14, 1845–1856. [Google Scholar] [CrossRef]
- Challagundla, K.B.; Wise, P.M.; Neviani, P.; Chava, H.; Murtadha, M.; Xu, T.; Kennedy, R.; Ivan, C.; Zhang, X.; Vannini, I.; et al. Exosome-Mediated Transfer of microRNAs Within the Tumor Microenvironment and Neuroblastoma Resistance to Chemotherapy. J. Natl. Cancer Inst. 2015, 107, djv135. [Google Scholar] [CrossRef] [Green Version]
- Caivano, A.; Laurenzana, I.; De Luca, L.; La Rocca, F.; Simeon, V.; Trino, S.; D’Auria, F.; Traficante, A.; Maietti, M.; Izzo, T.; et al. High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumor Biol. 2015, 36, 9739–9752. [Google Scholar] [CrossRef]
- Bouvy, C.; Wannez, A.; Laloy, J.; Chatelain, C.; Dogné, J.M. Transfer of multidrug resistance among acute myeloid leukemia cells via extracellular vesicles and their microRNA cargo. Leuk. Res. 2017, 62, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bello-Morales, R.; Ripa, I.; López-Guerrero, J.A. Extracellular vesicles in viral spread and antiviral response. Viruses 2020, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.; Rezaie, J.; Nouri, M.; Panahi, Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. 2020, 85, 104422. [Google Scholar] [CrossRef]
- Burton, J.O.; Hamali, H.A.; Singh, R.; Abbasian, N.; Parsons, R.; Patel, A.K.; Goodall, A.H.; Brunskill, N.J. Elevated Levels of Procoagulant Plasma Microvesicles in Dialysis Patients. PLoS ONE 2013, 8, e72663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buendía, P.; De Oca, A.M.; Madueño, J.A.; Merino, A.; Martín-Malo, A.; Aljama, P.; Ramírez, R.; Rodríguez, M.; Carracedo, J. Endothelial microparticles mediate inflammation-induced vascular calcification. FASEB J. 2015, 29, 173–181. [Google Scholar] [CrossRef]
- Amabile, N.; Guérin, A.P.; Leroyer, A.; Mallat, Z.; Nguyen, C.; Boddaert, J.; London, G.M.; Tedgui, A.; Boulanger, C.M. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J. Am. Soc. Nephrol. 2005, 16, 3381–3388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, Y.C.; Xiong, Q.; Blann, A.D.; Lip, G.Y.H. Relationship between renal function and circulating microparticles, soluble P-selectin and E-selectin levels in atrial fibrillation. J. Thromb. Thrombolysis 2017, 43, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Aufricht, C.; Beelen, R.; Eberl, M.; Fischbach, M.; Fraser, D.; Jörres, A.; Kratochwill, K.; LópezCabrera, M.; Rutherford, P.; Schmitt, C.P.; et al. Biomarker research to improve clinical outcomes of peritoneal dialysis: Consensus of the European Training and Research in Peritoneal Dialysis (EuTRiPD) network. Kidney Int. 2017, 92, 824–835. [Google Scholar] [CrossRef] [Green Version]
- Pearson, L.J.; Klaharn, I.Y.; Thongsawang, B.; Manuprasert, W.; Saejew, T.; Somparn, P.; Chuengsaman, P.; Kanjanabuch, T.; Pisitkun, T. Multiple extracellular vesicle types in peritoneal dialysis effluent are prominent and contain known biomarkers. PLoS ONE 2017, 12, e0178601. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Khan, K.; Kim, J.H. Biogenesis, membrane trafficking, functions, and next generation nanotherapeutics medicine of extracellular vesicles. Int. J. Nanomed. 2021, 16, 3357–3383. [Google Scholar] [CrossRef] [PubMed]
- Favretto, G.; da Cunha, R.S.; Dalboni, M.A.; de Oliveira, R.B.; Barreto, F.D.C.; Massy, Z.A.; Stinghen, A.E.M. Endothelial microparticles in uremia: Biomarkers and potential therapeutic targets. Toxins 2019, 11, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugel, B.; Martínez, M.C.; Kunzelmann, C.; Freyssinet, J.M. Membrane microparticles: Two sides of the coin. Physiology 2005, 20, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef]
- Amabile, N.; Guérin, A.P.; Tedgui, A.; Boulanger, C.M.; London, G.M. Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: A pilot study. Nephrol. Dial. Transplant. 2012, 27, 1873–1880. [Google Scholar] [CrossRef] [Green Version]
- Erdbrügger, U.; Le, T.H. Extracellular Vesicles in Renal Diseases: More than Novel Biomarkers? J. Am. Soc. Nephrol. 2016, 27, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Burger, D.; Montezano, A.C.; Nishigaki, N.; He, Y.; Carter, A.; Touyz, R.M. Endothelial microparticle formation by angiotensin II is mediated via ang II receptor type I/NADPH Oxidase/rho kinase pathways targeted to lipid rafts. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1898–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mause, S.F.; Weber, C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010, 107, 1047–1057. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Huber, L.C.; Hueber, A.J.; Reich, C.F.; Gay, S.; Distler, O.; Pisetsky, D.S. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis 2005, 10, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef] [Green Version]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular vesicles—connecting kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, K.C.; Bond, D.T.; McKee, M.; Skog, J.; Păunescu, T.G.; Da Silva, N.; Brown, D.; Russo, L.M. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 2010, 78, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Calero, L.; Martin-Lorenzo, M.; Alvarez-Llamas, G. Exosomes: A Potential Key Target in Cardio-Renal Syndrome. Front. Immunol. 2014, 5, 465. [Google Scholar] [CrossRef] [Green Version]
- Salih, M.; Zietse, R.; Hoorn, E.J. Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am. J. Physiol. Physiol. 2014, 306, F1251–F1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Pisitkun, T.; Aponte, A.; Yuen, P.S.T.; Hoffert, J.D.; Yasuda, H.; Hu, X.; Chawla, L.; Shen, R.F.; Knepper, M.A.; et al. Exosomal Fetuin-A identified by proteomics: A novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006, 70, 1847–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Street, J.M.; Birkhoff, W.; Menzies, R.I.; Webb, D.J.; Bailey, M.A.; Dear, J.W. Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. J. Physiol. 2011, 589, 6119–6127. [Google Scholar] [CrossRef]
- Sonoda, H.; Yokota-Ikeda, N.; Oshikawa, S.; Kanno, Y.; Yoshinaga, K.; Uchida, K.; Ueda, Y.; Kimiya, K.; Uezono, S.; Ueda, A.; et al. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am. J. Physiol. Physiol. 2009, 297, F1006–F1016. [Google Scholar] [CrossRef] [Green Version]
- Zubiri, I.; Posada-Ayala, M.; Sanz-Maroto, A.; Calvo, E.; Martin-Lorenzo, M.; Gonzalez-Calero, L.; de la Cuesta, F.; Lopez, J.A.; Fernandez-Fernandez, B.; Ortiz, A.; et al. Diabetic nephropathy induces changes in the proteome of human urinary exosomes as revealed by label-free comparative analysis. J. Proteom. 2014, 96, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Suazo, C.; Boltansky, A.; Ursu, M.; Carvajal, D.; Innocenti, G.; Vukusich, A.; Hurtado, M.; Villanueva, S.; Carreño, J.E.; et al. Urinary Exosomes as a Source of Kidney Dysfunction Biomarker in Renal Transplantation. Transplant. Proc. 2013, 45, 3719–3723. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.S.; Kim, E.; Bae, Y.-U.; Yang, W.M.; Lee, H.; Kim, H.; Noh, H.; Han, D.C.; Ryu, S.; Kwon, S.H. microRNA in Extracellular Vesicles Released by Damaged Podocytes Promote Apoptosis of Renal Tubular Epithelial Cells. Cells 2020, 9, 1409. [Google Scholar] [CrossRef]
- Meijers, B.K.I.; Van kerckhoven, S.; Verbeke, K.; Dehaen, W.; Vanrenterghem, Y.; Hoylaerts, M.F.; Evenepoel, P. The Uremic Retention Solute p-Cresyl Sulfate and Markers of Endothelial Damage. Am. J. Kidney Dis. 2009, 54, 891–901. [Google Scholar] [CrossRef]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Bellin, G.; Gardin, C.; Ferroni, L.; Chachques, J.; Rogante, M.; Mitrečić, D.; Ferrari, R.; Zavan, B. Exosome in Cardiovascular Diseases: A Complex World Full of Hope. Cells 2019, 8, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldenström, A.; Gennebäck, N.; Hellman, U.; Ronquist, G. Cardiomyocyte Microvesicles Contain DNA/RNA and Convey Biological Messages to Target Cells. PLoS ONE 2012, 7, e34653. [Google Scholar] [CrossRef] [Green Version]
- Nie, X.; Fan, J.; Li, H.; Yin, Z.; Zhao, Y.; Dai, B.; Dong, N.; Chen, C.; Wang, D.W. miR-217 Promotes Cardiac Hypertrophy and Dysfunction by Targeting PTEN. Mol. Ther. Nucleic Acids 2018, 12, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, N.A.; Ontoria-Oviedo, I.; González-King, H.; Diez-Juan, A.; Sepúlveda, P. Glucose Starvation in Cardiomyocytes Enhances Exosome Secretion and Promotes Angiogenesis in Endothelial Cells. PLoS ONE 2015, 10, e0138849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosme, J.; Guo, H.; Hadipour-Lakmehsari, S.; Emili, A.; Gramolini, A.O. Hypoxia-Induced Changes in the Fibroblast Secretome, Exosome, and Whole-Cell Proteome Using Cultured, Cardiac-Derived Cells Isolated from Neonatal Mice. J. Proteome Res. 2017, 16, 2836–2847. [Google Scholar] [CrossRef] [PubMed]
- Bang, C.; Batkai, S.; Dangwal, S.; Gupta, S.K.; Foinquinos, A.; Holzmann, A.; Just, A.; Remke, J.; Zimmer, K.; Zeug, A.; et al. Cardiac fibroblast–derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Investig. 2014, 124, 2136–2146. [Google Scholar] [CrossRef]
- Tian, C.; Gao, L.; Zimmerman, M.C.; Zucker, I.H. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am. J. Physiol. Circ. Physiol. 2018, 314, H928–H939. [Google Scholar] [CrossRef]
- Li, H.; Liao, Y.; Gao, L.; Zhuang, T.; Huang, Z.; Zhu, H.; Ge, J. Coronary serum exosomes derived from patients with myocardial ischemia regulate angiogenesis through the miR-939-mediated nitric oxide signaling pathway. Theranostics 2018, 8, 2079–2093. [Google Scholar] [CrossRef] [PubMed]
- Levin-Schwartz, Y.; Curtin, P.; Flores, D.; Aushev, V.N.; Tamayo-Ortiz, M.; Svensson, K.; Pantic, I.; Estrada-Gutierrez, G.; Pizano-Zárate, M.L.; Gennings, C.; et al. Exosomal miRNAs in urine associated with children’s cardiorenal parameters: A cross-sectional study. Epigenomics 2021, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Santelli, A.; Sun, I.O.; Eirin, A.; Abumoawad, A.M.; Woollard, J.R.; Lerman, A.; Textor, S.C.; Puranik, A.S.; Lerman, L.O. Senescent Kidney Cells in Hypertensive Patients Release Urinary Extracellular Vesicles. J. Am. Heart Assoc. 2019, 8, e012584. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.I.; Anderson, U.D.; Jayachandran, M.; Weissgerber, T.L.; Zand, L.; White, W.M.; Milic, N.; Suarez, M.L.G.; Vallapureddy, R.R.; Nääv, Å.; et al. Urinary Extracellular Vesicles of Podocyte Origin and Renal Injury in Preeclampsia. J. Am. Soc. Nephrol. 2017, 28, 3363–3372. [Google Scholar] [CrossRef] [PubMed]
- Dursun, I.; Poyrazoglu, H.M.; Gunduz, Z.; Ulger, H.; Yýkýlmaz, A.; Dusunsel, R.; Patýroglu, T.; Gurgoze, M. The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease. Nephrol. Dial. Transplant. 2009, 24, 2511–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crescitelli, R.; Lässer, C.; Lötvall, J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat. Protoc. 2021, 16, 1548–1580. [Google Scholar] [CrossRef] [PubMed]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Deun, J.; Mestdagh, P.; Sormunen, R.; Cocquyt, V.; Vermaelen, K.; Vandesompele, J.; Bracke, M.; De Wever, O.; Hendrix, A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles 2014, 3, 24858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Greene, J.A.; Hernández-Ortega, K.; Quiroz-Baez, R.; Resendis-Antonio, O.; Pichardo-Casas, I.; Sinclair, D.A.; Budnik, B.; Hidalgo-Miranda, A.; Uribe-Querol, E.; Ramos-Godínez, M.D.P.; et al. Quantitative proteomic analysis of extracellular vesicle subgroups isolated by an optimized method combining polymer-based precipitation and size exclusion chromatography. J. Extracell. Vesicles 2021, 10, e12087. [Google Scholar] [CrossRef] [PubMed]
- Onódi, Z.; Pelyhe, C.; Terézia Nagy, C.; Brenner, G.B.; Almási, L.; Kittel, Á.; Manček-Keber, M.; Ferdinandy, P.; Buzás, E.I.; Giricz, Z. Isolation of High-Purity Extracellular Vesicles by the Combination of Iodixanol Density Gradient Ultracentrifugation and Bind-Elute Chromatography From Blood Plasma. Front. Physiol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Veziroglu, E.M.; Mias, G.I. Characterizing Extracellular Vesicles and Their Diverse RNA Contents. Front. Genet. 2020, 11, 1–30. [Google Scholar] [CrossRef]
- Taylor, D.D.; Shah, S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Trachtenberg, A.J.; Hochberg, F.H.; Skog, J.; Kuo, W.P. Impact of Biofluid Viscosity on Size and Sedimentation Efficiency of the Isolated Microvesicles. Front. Physiol. 2012, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef] [PubMed]
- Cvjetkovic, A.; Lötvall, J.; Lässer, C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J. Extracell. Vesicles 2014, 3, 23111. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef]
- Duong, P.; Chung, A.; Bouchareychas, L.; Raffai, R.L. Cushioned-Density Gradient Ultracentrifugation (C-DGUC) improves the isolation efficiency of extracellular vesicles. PLoS ONE 2019, 14, e0215324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, C.; Li, T.; Liu, Z.; Li, L. Comparison of ultracentrifugation and density gradient separation methods for isolating Tca8113 human tongue cancer cell line-derived exosomes. Oncol. Lett. 2014, 8, 1701–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Araùjo, M.E.G.; Hube, L.A.; Stasyk, T. Isolation of endocitic organelles by density gradient centrifugation. In 2D PAGE: Sample Preparation and Fractionation; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2008; Volume 424, pp. 317–331. ISBN 978-1-60327-064-9. [Google Scholar]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—Efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef]
- Baranyai, T.; Herczeg, K.; Onódi, Z.; Voszka, I.; Módos, K.; Marton, N.; Nagy, G.; Mäger, I.; Wood, M.J.; El Andaloussi, S.; et al. Isolation of exosomes from blood plasma: Qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS ONE 2015, 10, e0145686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Nordin, J.Z.; Lee, Y.; Vader, P.; Mäger, I.; Johansson, H.J.; Heusermann, W.; Wiklander, O.P.B.; Hällbrink, M.; Seow, Y.; Bultema, J.J.; et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 879–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.J.; Becker, M.; Wen Wen, S.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Deville, S.; Berckmans, P.; Van Hoof, R.; Lambrichts, I.; Salvati, A.; Nelissen, I. Comparison of extracellular vesicle isolation and storage methods using high-sensitivity flow cytometry. PLoS ONE 2021, 16, e0245835. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Lee, J.C.; Byeon, S.K.; Rha, K.H.; Moon, M.H. Size Dependent Lipidomic Analysis of Urinary Exosomes from Patients with Prostate Cancer by Flow Field-Flow Fractionation and Nanoflow Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2017, 89, 2488–2496. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Simpson, R.J.; Greening, D.W. A Protocol for Isolation and Proteomic Characterization of Distinct Extracellular Vesicle Subtypes by Sequential Centrifugal Ultrafiltration. In Exosomes and Microvesicles; Hill, A.F., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1545, ISBN 978-1-4939-6726-1. [Google Scholar]
- Chuo, S.T.Y.; Chien, J.C.Y.; Lai, C.P.K. Imaging extracellular vesicles: Current and emerging methods. J. Biomed. Sci. 2018, 25, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Malenica, M.; Vukomanović, M.; Kurtjak, M.; Masciotti, V.; dal Zilio, S.; Greco, S.; Lazzarino, M.; Krušić, V.; Perčić, M.; Jelovica Badovinac, I.; et al. Perspectives of Microscopy Methods for Morphology Characterisation of Extracellular Vesicles from Human Biofluids. Biomedicines 2021, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Mun, J.Y. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J. Vis. Exp. 2018, 2018, 5–9. [Google Scholar] [CrossRef]
- Yuana, Y.; Koning, R.I.; Kuil, M.E.; Rensen, P.C.N.; Koster, A.J.; Bertina, R.M.; Osanto, S. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J. Extracell. Vesicles 2013, 2, 21494. [Google Scholar] [CrossRef] [PubMed]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The methods of choice for extracellular vesicles (EVs) characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef] [PubMed]
- Mehdiani, A.; Maier, A.; Pinto, A.; Barth, M.; Akhyari, P.; Lichtenberg, A. An innovative method for exosome quantification and size measurement. J. Vis. Exp. 2015, 95, e50974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef] [PubMed]
- Hartjes, T.A.; Mytnyk, S.; Jenster, G.W.; van Steijn, V.; van Royen, M.E. Extracellular vesicle quantification and characterization: Common methods and emerging approaches. Bioengineering 2019, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Chandler, W.L.; Yeung, W.; Tait, J.F. A new microparticle size calibration standard for use in measuring smaller microparticles using a new flow cytometer. J. Thromb. Haemost. 2011, 9, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pol, E.; Van Gemert, M.J.C.; Sturk, A.; Nieuwland, R.; Van Leeuwen, T.G. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J. Thromb. Haemost. 2012, 10, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Fraser, K.; Ghaddar, B.; Yang, K.; Kim, E.; Balaj, L.; Chiocca, E.A.; Breakefield, X.O.; Lee, H.; Weissleder, R. Multiplexed Profiling of Single Extracellular Vesicles. ACS Nano 2018, 12, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Kim, D.K.; Kim, Y.K.; Gho, Y.S. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom. Rev. 2015, 34, 474–490. [Google Scholar] [CrossRef]
- Subedi, P.; Schneider, M.; Philipp, J.; Azimzadeh, O.; Metzger, F.; Moertl, S.; Atkinson, M.J.; Tapio, S. Comparison of methods to isolate proteins from extracellular vesicles for mass spectrometry-based proteomic analyses. Anal. Biochem. 2019, 584, 113390. [Google Scholar] [CrossRef]
- Gidlöf, O.; Evander, M.; Rezeli, M.; Marko-Varga, G.; Laurell, T.; Erlinge, D. Proteomic profiling of extracellular vesicles reveals additional diagnostic biomarkers for myocardial infarction compared to plasma alone. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Turchinovich, A.; Drapkina, O.; Tonevitsky, A. Transcriptome of extracellular vesicles: State-of-the-art. Front. Immunol. 2019, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeringer, E.; Barta, T.; Schageman, J.; Cheng, A.; Vlassov, A.V. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130502. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Batagov, A.O.; Schinelli, S.; Wang, J.; Wang, Y.; El Fatimy, R.; Rabinovsky, R.; Balaj, L.; Chen, C.C.; Hochberg, F.; et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat. Commun. 2017, 8, 1145. [Google Scholar] [CrossRef] [Green Version]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez García, G.; Galicia García, G.; Zalapa Soto, J.; Izquierdo Medina, A.; Rotzinger-Rodríguez, M.; Casas Aguilar, G.A.; López Pacheco, C.P.; Aguayo, Á.; Aguilar-Hernandez, M.M. Analysis of RNA yield in extracellular vesicles isolated by membrane affinity column and differential ultracentrifugation. PLoS ONE 2020, 15, e0238545. [Google Scholar] [CrossRef]
- Tang, Y.T.; Huang, Y.Y.; Zheng, L.; Qin, S.H.; Xu, X.P.; An, T.X.; Xu, Y.; Wu, Y.S.; Hu, X.M.; Ping, B.H.; et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int. J. Mol. Med. 2017, 40, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Everaert, C.; Helsmoortel, H.; Decock, A.; Hulstaert, E.; Van Paemel, R.; Verniers, K.; Nuytens, J.; Anckaert, J.; Nijs, N.; Tulkens, J.; et al. Performance assessment of total RNA sequencing of human biofluids and extracellular vesicles. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; He, X.; Le, T.; Carnino, J.M.; Jin, Y. Single-step RT-qPCR for detection of extracellular vesicle microRNAs in vivo: A time- and cost-effective method. Am. J. Physiol. Cell. Mol. Physiol. 2020, 318, L742–L749. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.A.; Münster-Wandowski, A.; Nonnenmacher, A.; Keilholz, U.; Liebs, S. Analysis of cancer-related mutations in extracellular vesicles RNA by Droplet DigitalTM PCR. Biotechniques 2020, 69, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yan, I.K.; Kim, C.; Kim, J.; Patel, T. Analysis of Extracellular RNA by Digital PCR. Front. Oncol. 2014, 4, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Moldovan, L.; Batte, K.; Wang, Y.; Wisler, J.; Piper, M. Analyzing the circulating microRNAs in exosomes/extracellular vesicles from serum or plasma by qRT-PCR. In Circulating MicroRNAs; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1024, pp. 129–145. ISBN 9781627034524. [Google Scholar]
- Hough, K.P.; Wilson, L.S.; Trevor, J.L.; Strenkowski, J.G.; Maina, N.; Kim, Y.-I.; Spell, M.L.; Wang, Y.; Chanda, D.; Dager, J.R.; et al. Unique Lipid Signatures of Extracellular Vesicles from the Airways of Asthmatics. Sci. Rep. 2018, 8, 10340. [Google Scholar] [CrossRef]
- Burrello, J.; Biemmi, V.; Dei Cas, M.; Amongero, M.; Bolis, S.; Lazzarini, E.; Bollini, S.; Vassalli, G.; Paroni, R.; Barile, L. Sphingolipid composition of circulating extracellular vesicles after myocardial ischemia. Sci. Rep. 2020, 10, 16182. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- Wang, G.; Dinkins, M.; He, Q.; Zhu, G.; Poirier, C.; Campbell, A.; Mayer-Proschel, M.; Bieberich, E. Astrocytes Secrete Exosomes Enriched with Proapoptotic Ceramide and Prostate Apoptosis Response 4 (PAR-4). J. Biol. Chem. 2012, 287, 21384–21395. [Google Scholar] [CrossRef] [Green Version]
- Rabia, M.; Leuzy, V.; Soulage, C.; Durand, A.; Fourmaux, B.; Errazuriz-Cerda, E.; Köffel, R.; Draeger, A.; Colosetti, P.; Jalabert, A.; et al. Bis(monoacylglycero)phosphate, a new lipid signature of endosome-derived extracellular vesicles. Biochimie 2020, 178, 26–38. [Google Scholar] [CrossRef]
- Singhto, N.; Vinaiphat, A.; Thongboonkerd, V. Discrimination of urinary exosomes from microvesicles by lipidomics using thin layer liquid chromatography (TLC) coupled with MALDI-TOF mass spectrometry. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vorselen, D.; van Dommelen, S.M.; Sorkin, R.; Piontek, M.C.; Schiller, J.; Döpp, S.T.; Kooijmans, S.A.A.; van Oirschot, B.A.; Versluijs, B.A.; Bierings, M.B.; et al. The fluid membrane determines mechanics of erythrocyte extracellular vesicles and is softened in hereditary spherocytosis. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Datta-Chaudhuri, A.; Deme, P.; Dickens, A.; Dastgheyb, R.; Bhargava, P.; Bi, H.; Haughey, N.J. Lipidomic characterization of extracellular vesicles in human serum. J. Circ. Biomark. 2019, 8, 184945441987984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.; Palviainen, M.; Reichardt, N.C.; Siljander, P.R.M.; Falcón-Pérez, J.M. Metabolomics applied to the study of extracellular vesicles. Metabolites 2019, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Clos-Garcia, M.; Loizaga-Iriarte, A.; Zuñiga-Garcia, P.; Sánchez-Mosquera, P.; Rosa Cortazar, A.; González, E.; Torrano, V.; Alonso, C.; Pérez-Cormenzana, M.; Ugalde-Olano, A.; et al. Metabolic alterations in urine extracellular vesicles are associated to prostate cancer pathogenesis and progression. J. Extracell. Vesicles 2018, 7, 1470442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; An, M.; Cuneo, K.C.; Lubman, D.M.; Li, L. High-Performance Chemical Isotope Labeling Liquid Chromatography Mass Spectrometry for Exosome Metabolomics. Anal. Chem. 2018, 90, 8314–8319. [Google Scholar] [CrossRef] [PubMed]
- Dudzik, D.; Macioszek, S.; Struck-Lewicka, W.; Kordalewska, M.; Buszewska-Forajta, M.; Waszczuk-Jankowska, M.; Wawrzyniak, R.; Artymowicz, M.; Raczak-Gutknecht, J.; Siluk, D.; et al. Perspectives and challenges in extracellular vesicles untargeted metabolomics analysis. TrAC Trends Anal. Chem. 2021, 143, 116382. [Google Scholar] [CrossRef]
- Balan, V.; Mihai, C.-T.; Cojocaru, F.-D.; Uritu, C.-M.; Dodi, G.; Botezat, D.; Gardikiotis, I. Vibrational Spectroscopy Fingerprinting in Medicine: From Molecular to Clinical Practice. Materials 2019, 12, 2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, J.M.; Butler, H.J.; Palmer, D.S.; Baker, M.J. Biofluid spectroscopic disease diagnostics: A review on the processes and spectral impact of drying. J. Biophotonics 2018, 11, e201700299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuiblet, V.; Fere, M.; Gobinet, C.; Birembaut, P.; Piot, O.; Rieu, P. Renal Graft Fibrosis and Inflammation Quantification by an Automated Fourier–Transform Infrared Imaging Technique. J. Am. Soc. Nephrol. 2016, 27, 2382–2391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Ramoji, A.; Guo, S.; Bocklitz, T.; Boivin-Jahns, V.; Möller, J.; Kiehntopf, M.; Noutsias, M.; Popp, J.; Neugebauer, U. Vibrational spectroscopy as a powerful tool for follow-up immunoadsorption therapy treatment of dilated cardiomyopathy—A case report. Analyst 2020, 145, 486–496. [Google Scholar] [CrossRef]
- Nepomuceno, G.; Junho, C.V.C.; Carneiro-Ramos, M.S.; da Silva Martinho, H. Tyrosine and Tryptophan vibrational bands as markers of kidney injury: A renocardiac syndrome induced by renal ischemia and reperfusion study. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Szentirmai, V.; Wacha, A.; Németh, C.; Kitka, D.; Rácz, A.; Héberger, K.; Mihály, J.; Varga, Z. Reagent-free total protein quantification of intact extracellular vesicles by attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy. Anal. Bioanal. Chem. 2020, 412, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Paolini, L.; Federici, S.; Consoli, G.; Arceri, D.; Radeghieri, A.; Alessandri, I.; Bergese, P. Fourier-transform Infrared (FT-IR) spectroscopy fingerprints subpopulations of extracellular vesicles of different sizes and cellular origin. J. Extracell. Vesicles 2020, 9, 1741174. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Denomination | Description |
|---|---|
| Type I | Heart failure causing acute kidney injury |
| Type II | Chronic heart failure causing chronic kidney disease |
| Type III | Acute kidney injury causing acute heart disease |
| Type IV | Chronic kidney disease causing chronic heart failure |
| Type V | Systemic condition causing heart and kidney disease |
| Reference | n | EVs Type | Methodology |
|---|---|---|---|
| [72] | 8 | Microvesicles | Electron microscopy, nanoparticle tracking analysis (NTA), flow cytometry, procoagulant activity, and Western blot. |
| [92] | 13 | Multiple extracellular vesicles | Isolation by differential centrifugation and size exclusion chromatography. Vesicle analysis by electron microscopy, NTA, dynamic lighting scattering (DLS), and tandem mass spectrometry. |
| [57,65] | 11 | Multiple extracellular vesicles | Extracellular vesicles were isolated by size exclusion chromatography, and proteomics was analyzed by mass spectrometry (LC-MS/MS) |
| [66] | 30 | Exosomes containing Aquaporin 1 (AQP1) | Exosomes were isolated by differential centrifugation and identified by Western Blot. Expression of AQP1 in mesothelial cells was done by immunofluorescence. The quantification of AQP1 in the dialysate was performed using a commercial enzyme-linked immunosorbent assay (ELISA) kit. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, C.A.B.; da Cunha, R.S.; Junho, C.V.C.; da Silva, J.V.; Moreno-Amaral, A.N.; de Moraes, T.P.; Carneiro-Ramos, M.S.; Stinghen, A.E.M. Extracellular Vesicles and Their Relationship with the Heart–Kidney Axis, Uremia and Peritoneal Dialysis. Toxins 2021, 13, 778. https://doi.org/10.3390/toxins13110778
Azevedo CAB, da Cunha RS, Junho CVC, da Silva JV, Moreno-Amaral AN, de Moraes TP, Carneiro-Ramos MS, Stinghen AEM. Extracellular Vesicles and Their Relationship with the Heart–Kidney Axis, Uremia and Peritoneal Dialysis. Toxins. 2021; 13(11):778. https://doi.org/10.3390/toxins13110778
Chicago/Turabian StyleAzevedo, Carolina Amaral Bueno, Regiane Stafim da Cunha, Carolina Victoria Cruz Junho, Jessica Verônica da Silva, Andréa N. Moreno-Amaral, Thyago Proença de Moraes, Marcela Sorelli Carneiro-Ramos, and Andréa Emilia Marques Stinghen. 2021. "Extracellular Vesicles and Their Relationship with the Heart–Kidney Axis, Uremia and Peritoneal Dialysis" Toxins 13, no. 11: 778. https://doi.org/10.3390/toxins13110778
APA StyleAzevedo, C. A. B., da Cunha, R. S., Junho, C. V. C., da Silva, J. V., Moreno-Amaral, A. N., de Moraes, T. P., Carneiro-Ramos, M. S., & Stinghen, A. E. M. (2021). Extracellular Vesicles and Their Relationship with the Heart–Kidney Axis, Uremia and Peritoneal Dialysis. Toxins, 13(11), 778. https://doi.org/10.3390/toxins13110778