Effect of Botulinum Toxin Injection on the Progression of Hip Dislocation in Patients with Spastic Cerebral Palsy: A Pilot Study
Abstract
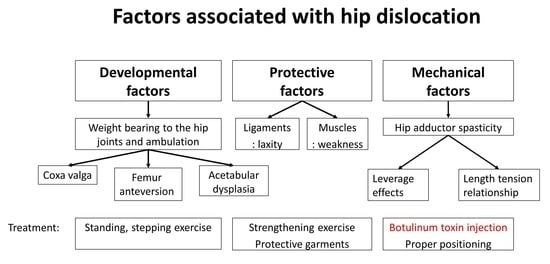
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Participants
5.2. Procedures
5.3. Outcomes
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robin, J.; Kerr Graham, H.; Selber, P.; Dobson, F.; Smith, K.; Baker, R. Proximal femoral geometry in cerebral palsy: A population-based cross-sectional study. J. Bone Jt. Surg.—Ser. B 2008, 90, 1372–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, M.K.; Zulkarnain, A.; Lee, J.B.; Cho, B.C.; Chung, C.Y.; Lee, K.M.; Sung, K.H.; Park, M.S. Functional status and amount of hip displacement independently affect acetabular dysplasia in cerebral palsy. Dev. Med. Child Neurol. 2017, 59, 743–749. [Google Scholar] [CrossRef]
- Valencia, F.G. Management of Hip deformities in cerebral palsy. Orthop. Clin. N. Am. 2010, 41, 549–559. [Google Scholar] [CrossRef]
- Kim, I.S.; Park, D.; Ko, J.Y.; Ryu, J.S. Are Seating Systems with a Medial Knee Support Really Helpful for Hip Displacement in Children with Spastic Cerebral Palsy GMFCS IV and V? Arch. Phys. Med. Rehabil. 2019, 100, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, D.; Ko, J.Y.; Park, Y.; Yoon, Y.H.; Suh, J.H.; Ryu, J.S. The Mechanism of Hip Dislocation Related to the Use of Abduction Bar and Hip Compression Bandage in Patients with Spastic Cerebral Palsy. Am. J. Phys. Med. Rehabil. 2019, 98, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.A. Kinesiology of the hip: A focus on muscular actions. J. Orthop. Sports Phys. Ther. 2010, 40, 82–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobl, W.; Theologis, T.; Brunner, R.; Kocer, S.; Viehweger, E.; Pascual-Pascual, I.; Placzek, R. Best clinical practice in botulinum toxin treatment for children with cerebral palsy. Toxins 2015, 7, 1629–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, S.D.; Juricic, M.; Hesketh, K.; Mclean, L.; Magnuson, S.; Gasior, S.; Schaeffer, E.; O’donnell, M.; Mulpuri, K. Prevention of hip displacement in children with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2017, 59, 1130–1138. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.J.; Rha, D.W.; Kim, H.W.; Park, E.S. Comparison of Botulinum Toxin Type A Injection and Soft-Tissue Surgery to Treat Hip Subluxation in Children with Cerebral Palsy. Arch. Phys. Med. Rehabil. 2008, 89, 2108–2113. [Google Scholar] [CrossRef] [PubMed]
- Placzek, R.; Deuretzbacher, G.; Meiss, A.L. Treatment of Lateralisation and Subluxation of the Hip in Cerebral Palsy with Botulinum Toxin A: Preliminary Results Based on the Analysis of Migration Percentage Data. Neuropediatrics 2004, 35, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Pidcock, F.S.; Fish, D.E.; Johnson-Greene, D.; Borras, I.; McGready, J.; Silberstein, C.E. Hip migration percentage in children with cerebral palsy treated with botulinum toxin type A. Arch. Phys. Med. Rehabil. 2005, 86, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.H.; Heinen, F.; Westhoff, B.; Doederlein, L.; Reissig, A.; Berweck, S.; Linder-Lucht, M.; Schandelmaier, S.; Mall, V. Hip lateralisation in children with bilateral spastic cerebral palsy treated with botulinum toxin type A: A 2-year follow-up. Neuropediatrics 2011, 42, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, K.; Ang, S.G.; Thomason, P.; Graham, H.K. The impact of botulinum toxin A and abduction bracing on long-term hip development in children with cerebral palsy. Dev. Med. Child Neurol. 2012, 54, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.K.; Boyd, R.; Carlin, J.B.; Dobson, F.; Lowe, K.; Nattrass, G.; Thomason, P.; Wolfe, R.; Reddihough, D. Does botulinum toxin A combined with bracing prevent hip displacement in children with cerebral palsy and “hips at risk”? A randomized, controlled trial. J. Bone Jt. Surg.—Ser. A 2008, 90, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-Y.; Chung, C.-H.; Matthews, D.J.; Chu, H.-Y.; Chen, L.-C.; Yang, S.-S.; Chien, W.-C. Long-term effect of botulinum toxin A on the hip and spine in cerebral palsy: A national retrospective cohort study in Taiwan. PLoS ONE 2021, 16, e0255143. [Google Scholar] [CrossRef]
- Multani, I.; Manji, J.; Hastings-Ison, T.; Khot, A.; Graham, K. Botulinum Toxin in the Management of Children with Cerebral Palsy. Pediatr. Drugs 2019, 21, 261–281. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, A.S.; Ertl-Wagner, B.; Britsch, S.; Schröder, J.M.; Nikolin, S.; Weis, J.; Müller-Felber, W.; Koerte, I.; Stehr, M.; Berweck, S.; et al. Muscle biopsy substantiates long-term MRI alterations one year after a single dose of botulinum toxin injected into the lateral gastrocnemius muscle of healthy volunteers. Mov. Disord. 2009, 24, 1494–1503. [Google Scholar] [CrossRef]
- Koerte, I.K.; Schroeder, A.S.; Fietzek, U.M.; Borggraefe, I.; Kerscher, M.; Berweck, S.; Reiser, M.; Ertl-Wagner, B.; Heinen, F. Muscle atrophy beyond the clinical effect after a single dose of onabotulinumtoxina injected in the procerus muscle: A study with magnetic resonance imaging. Dermatol. Surg. 2013, 39, 761–765. [Google Scholar] [CrossRef]
- Multani, I.; Manji, J.; Tang, M.J.; Herzog, W.; Howard, J.J.; Graham, H.K. Sarcopenia, cerebral palsy, and botulinum toxin type A. JBJS Rev. 2019, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- van den Noort, J.C.; Bar-On, L.; Aertbeliën, E.; Bonikowski, M.; Braendvik, S.M.; Broström, E.W.; Buizer, A.I.; Burridge, J.H.; van Campenhout, A.; Dan, B.; et al. European consensus on the concepts and measurement of the pathophysiological neuromuscular responses to passive muscle stretch. Eur. J. Neurol. 2017, 24, 981-e38. [Google Scholar] [CrossRef]
- Latash, M.L. Muscle coactivation: Definitions, mechanisms, and functions. J. Neurophysiol. 2018, 120, 88–104. [Google Scholar] [CrossRef]
- Rice, J.; Skuza, P.; Baker, F.; Russo, R.; Fehlings, D. Identification and measurement of dystonia in cerebral palsy. Dev. Med. Child Neurol. 2017, 59, 1249–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terjesen, T. The natural history of hip development in cerebral palsy. Dev. Med. Child Neurol. 2012, 54, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.H.; Kwon, S.S.; Narayanan, U.G.; Chung, C.Y.; Lee, K.M.; Lee, S.Y.; Lee, D.J.; Park, M.S. Transcultural adaptation and validation of the Korean version of Caregiver Priorities & Child Health Index of Life with Disabilities (CPCHILD). Disabil. Rehabil. 2015, 37, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Copeland, L.; Edwards, P.; Thorley, M.; Donaghey, S.; Gascoigne-Pees, L.; Kentish, M.; Cert, G.; Lindsley, J.; McLennan, K.; Sakzewski, L.; et al. Botulinum toxin a for nonambulatory children with cerebral palsy: A double blind randomized controlled trial. J. Pediatr. 2014, 165, 140–146.e4. [Google Scholar] [CrossRef]
- Mutlu, A.; Livanelioglu, A.; Gunel, M.K. Reliability of Ashworth and Modified Ashworth Scales in children with spastic cerebral palsy. BMC Musculoskelet. Disord. 2008, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Besomi, M.; Hodges, P.W.; Clancy, E.A.; Van Dieën, J.; Hug, F.; Lowery, M.; Merletti, R.; Søgaard, K.; Wrigley, T.; Besier, T.; et al. Consensus for experimental design in electromyography (CEDE) project: Amplitude normalization matrix. J. Electromyogr. Kinesiol. 2020, 53, 102438. [Google Scholar] [CrossRef]
- Hägglund, G.; Lauge-Pedersen, H.; Wagner, P. Characteristics of children with hip displacement in cerebral palsy. BMC Musculoskelet. Disord. 2007, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, P. The Definition and Classification of Cerebral Palsy: Are We Any Further Ahead in 2006? Neoreviews 2006, 7, e569–e574. [Google Scholar] [CrossRef]
- Manikowska, F.; Chen, B.P.J.; Jóźwiak, M.; Lebiedowska, M.K. The popliteal angle tests in patients with cerebral palsy. J. Pediatr. Orthop. Part B 2019, 28, 332–336. [Google Scholar] [CrossRef]
- AACPDM—Hip Surveillance in Cerebral Palsy. Available online: https://www.aacpdm.org/publications/care-pathways/hip-surveillance-in-cerebral-palsy (accessed on 6 December 2021).
- AusACPDM—Australian Hip Surveillance Guidelines. Available online: https://www.ausacpdm.org.au/resources/australian-hip-surveillance-guidelines/ (accessed on 6 December 2021).
- CHBC Hip Surveillance Program for Children with Cerebral Palsy. Available online: https://www.childhealthbc.ca/initiatives/chbc-hip-surveillance-program-children-cerebral-palsy (accessed on 6 December 2021).


| Variables | Intervention (n = 20) | Control * (n = 24) | p-Value ** |
|---|---|---|---|
| Age (yr) | 5.1 (1.86) | 6.13 (2.47) | 0.13 |
| Age group (yr) | |||
| 2–4 | 9 | 7 | |
| 4–6 | 5 | 5 | |
| 6–8 | 6 | 8 | |
| 8–10 | 0 | 4 | |
| Sex (M:F) | 14:6 | 13:11 | 0.29 |
| GMFCS level (IV:V) | 3:17 | 11:13 | <0.01 |
| Height (cm) | 100.6 (12.27) | ||
| Weight (kg) | 15.07 (4.41) | ||
| Hip Migration index | |||
| Right | 38.19 (22.53) | 30.70 (18.94) | 0.25 |
| Left | 39.14 (29.26) | 30.14 (14.77) | 0.22 |
| Hip and Knee ROM | |||
| Hip abduction (with hip 90’ flexion) | 29.5 (16.54) | ||
| Hip abduction (with hip extension) | 24.5 (15.64) | ||
| Knee flexion | 111 (63.73) | ||
| Knee extension (Popliteal angle) | 9.75 (14.19) | ||
| Hip Adductor spasticity (MAS) | |||
| <2 | 9 | ||
| ≥2 | 11 | ||
| Orthoses | |||
| Spinal orthoses | 0 | ||
| Postural support | 3 | ||
| Ankle foot orthoses | 8 | ||
| Anti-spasticity medication | 7 |
| Baseline | 1 Month | 2 Months | 3 Months | 6 Months | 7 Months | 12 Months | |
|---|---|---|---|---|---|---|---|
| Adductor longus m. | 61.62 (56.27) | 30.03 (24.18) * | 34.14 (32.76) | 32.79 (18.49) | 43.75 (35.20) | 42.18 (46.41) | 45.40 (51.67) |
| Adductor magnus m. | 55.90 (49.52) | 32.84 (34.65) * | 28.86 (23.28) * | 29.55 (20.36) * | 39.34 (23.56) | 23.32 (19.92) * | 35.80 (34.36) |
| Adductor muscles sum | 117.52 (102.28) | 62.87 (52.37) * | 63.00 (53.37) * | 62.35 (36.67) * | 83.09 (56.63) | 65.50 (63.97) * | 81.20 (83.87) |
| Tensor fascia lata m. | 78.55 (96.32) | 47.63 (39.77) | 50.21 (44.82) | 53.68 (63.52) | 72.64 (69.99) | 61.53 (50.36) | 69.25 (83.94) |
| Gluteus medius m. | 24.84 (24.37) | 27.37 (29.83) | 26.01 (32.20) | 25.21 (27.08) | 36.90 (26.89) | 34.17 (33.95) | 29.21 (27.05) |
| Abductor muscles sum | 103.39 (112.10) | 75.00 (61.93) | 76.22 (65.32) | 78.89 (87.86) | 109.54 (83.55) | 95.69 (75.56) | 98.46 (103.12) |
| Botox Injection (n = 14) | ICC 1 | Control 2 (n = 24) | |
|---|---|---|---|
| Hip migration index | |||
| Baseline | 31.84 (16.23) | 0.98 | 30.43 (16.85) |
| 6 months | 31.95 (13.69) | 0.97 | |
| 12 months | 31.80 (14.27) | 0.95 | |
| One year progression rate | −0.04 (8.63) | 3.27 (8.62) |
| Baseline | 1 Month | 2 Months | 3 Months | 6 Months | 7 Months | 12 Months | |
|---|---|---|---|---|---|---|---|
| ROM | |||||||
| Hip abduction (with hip 90’ flexion) | 29.50 (16.54) | 37.37 (12.4) | 36.94 (14.87) | 41.56 (7.90) * | 41.76 (7.06) * | 43.67 (3.52) * | 45.00 (0.00) * |
| Hip abduction (with hip extension) | 24.50 (15.64) | 37.37 (12.84) * | 34.17 (14.68) * | 40.31 (9.03) * | 30.29 (9.43) | 42.33 (5.63) * | 37.69 (10.33) |
| Knee flexion | 111.00 (63.73) | 135.79 (42.86) | 133.89 (46.92) | 144.06 (23.75) | 141.18 (36.38) | 150.00 (0.00) | 142.31 (27.74) |
| Knee extension (Popliteal angle) | 9.75 (14.19) | 0.79 (2.5) * | 0.83 (3.54) * | 7.50 (21.21) | 3.24 (10.15) | 0 (0) * | 2.31 (8.32) |
| Hip Adductor spasticity (MAS) | |||||||
| <2 ≥2 | 911 | 136 | 135 | 143 | 153 | 142 | 122 |
| CPCHILD | 25.44 (17.39) | 33.59 (17.64) * | 39.30 (19.94) * | 38.93 (20.14) * | 39.73 (22.00) * | 39.22 (17.56) * | 39.29 (13.62) * |
| Questionnaire (Likert scale) | |||||||
| Quality of life | 3.89 (0.81) | 3.44 (0.70) | 3.71 (0.92) | 3.61 (1.14) | 3.44 (0.73) | 3.14 (0.86) | |
| Comfort | 4.05 (0.91) | 3.50 (0.71) | 3.65 (0.86) | 3.56 (1.15) | 3.44 (0.73) | 3.29 (0.73) | |
| Ease of care | 4.05 (0.71) | 3.39 (0.70) | 3.65 (1.00) | 3.50 (1.15) | 3.38 (0.72) | 3.00 (0.78) | |
| Overall health | 3.95 (0.85) | 3.44 (0.70) | 3.71 (0.92) | 3.83 (0.79) | 3.25 (0.77) | 3.29 (0.47) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Lee, S.; Jang, J.; Lim, J.; Ryu, J.S. Effect of Botulinum Toxin Injection on the Progression of Hip Dislocation in Patients with Spastic Cerebral Palsy: A Pilot Study. Toxins 2021, 13, 872. https://doi.org/10.3390/toxins13120872
Lee Y, Lee S, Jang J, Lim J, Ryu JS. Effect of Botulinum Toxin Injection on the Progression of Hip Dislocation in Patients with Spastic Cerebral Palsy: A Pilot Study. Toxins. 2021; 13(12):872. https://doi.org/10.3390/toxins13120872
Chicago/Turabian StyleLee, Yookyung, Seungeun Lee, Joonyoung Jang, Jiwoon Lim, and Ju Seok Ryu. 2021. "Effect of Botulinum Toxin Injection on the Progression of Hip Dislocation in Patients with Spastic Cerebral Palsy: A Pilot Study" Toxins 13, no. 12: 872. https://doi.org/10.3390/toxins13120872
APA StyleLee, Y., Lee, S., Jang, J., Lim, J., & Ryu, J. S. (2021). Effect of Botulinum Toxin Injection on the Progression of Hip Dislocation in Patients with Spastic Cerebral Palsy: A Pilot Study. Toxins, 13(12), 872. https://doi.org/10.3390/toxins13120872