Solution Structures of Bacillus anthracis Protective Antigen Proteins Using Small Angle Neutron Scattering and Protective Antigen 63 Ion Channel Formation Kinetics
Abstract
:1. Introduction
2. Results
2.1. Solution Structures of PA83
2.2. Solution Structure of PA63
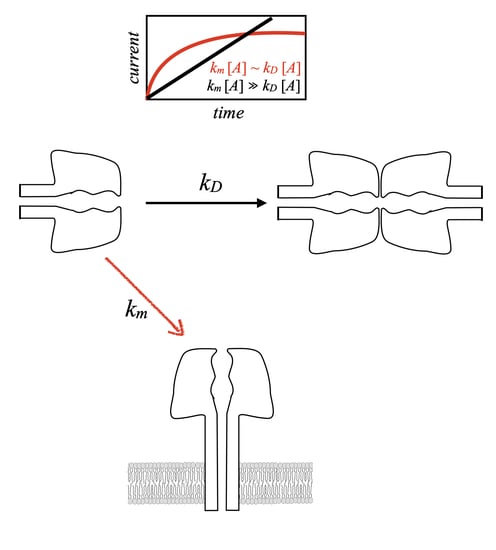
2.3. PA63 Channel Formation Kinetics Measurements
2.3.1. Channel Formation by B. anthracis PA63
2.3.2. AC Current Measurement
2.3.3. PA63 Channel Formation Kinetics
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Small Angle Neutron Scattering (SANS) of PA83 and PA63 Proteins in Solution
5.2. SANS Data Collection
5.3. SANS Curve Fitting
5.4. Electrophysiology: Membrane Formation and Channel Activity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Ashcroft, F.M. Ion Channels and Disease; Academic Press: Cambridge, MA, USA, 1999; ISBN 9780080535210. [Google Scholar]
- Kasianowicz, J.J. Introduction to Ion Channels and Disease. Chem. Rev. 2012, 112, 6215–6217. [Google Scholar] [CrossRef]
- Collier, R.J.; Young, J.A. Anthrax Toxin. Annu. Rev. Cell Dev. Biol. 2003, 19, 45–70. [Google Scholar] [CrossRef]
- Young, J.A.; Collier, R.J. Anthrax Toxin: Receptor Binding, Internalization, Pore Formation, and Translocation. Annu. Rev. Biochem. 2007, 76, 243–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, K.A.; Mogridge, J.; Mourez, M.; Collier, R.J.; Young, J.A.T. Identification of the Cellular Receptor for Anthrax Toxin. Nat. Cell Biol. 2001, 414, 225–229. [Google Scholar] [CrossRef]
- Gordon, V.M.; Klimpel, K.R.; Arora, N.; A Henderson, M.; Leppla, S.H. Proteolytic Activation of Bacterial Toxins by Eukaryotic Cells is Performed by Furin and by Additional Cellular Proteases. Infect. Immun. 1995, 63, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Milne, J.C.; Furlong, D.; Hanna, P.C.; Wall, J.S.; Collier, R.J. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J. Biol. Chem. 1994, 269, 20607–20612. [Google Scholar] [CrossRef]
- Milne, J.C.; Collier, R.J. pH-Dependent Permeabilization of the Plasma Membrane of Mammalian Cells by Anthrax Protective Antigen. Mol. Microbiol. 1993, 10, 647–653. [Google Scholar] [CrossRef] [PubMed]
- van der Goot, G.; Young, J.A. Receptors of Anthrax Toxin and Cell Entry. Mol. Asp. Med. 2009, 30, 406–412. [Google Scholar] [CrossRef] [Green Version]
- Ascenzi, P.; Visca, P.; Ippolito, G.; Spallarossa, A.; Bolognesi, M.; Montecucco, C. Anthrax Toxin: A Tripartite Lethal Combination. FEBS Lett. 2002, 531, 384–388. [Google Scholar] [CrossRef] [Green Version]
- Halverson, K.M.; Panchal, R.; Nguyen, T.L.; Gussio, R.; Little, S.F.; Misakian, M.; Bavari, S.; Kasianowicz, J.J. Anthrax Biosensor, Protective Antigen Ion Channel Asymmetric Blockade. J. Biol. Chem. 2005, 280, 34056–34062. [Google Scholar] [CrossRef] [Green Version]
- Panchal, R.G.; Halverson, K.M.; Ribot, W.; Lane, D.; Kenny, T.; Abshire, T.G.; Ezzell, J.W.; Hoover, T.A.; Powell, B.; Little, S.; et al. Purified Bacillus anthracis Lethal Toxin Complex Formed in vitro and During Infection Exhibits Functional and Biological Activity. J. Biol. Chem. 2005, 280, 10834–10839. [Google Scholar] [CrossRef] [Green Version]
- Neumeyer, T.; Tonello, F.; Molin, F.D.; Schiffler, B.; Benz, R. Anthrax Edema Factor, Voltage-dependent Binding to the Protective Antigen Ion Channel and Comparison to LF Binding. J. Biol. Chem. 2006, 281, 32335–32343. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Finkelstein, A.; Collier, R.J. Evidence that Translocation of Anthrax Toxin’s Lethal Factor is Initiated by Entry of its N Terminus into the Protective Antigen Channel. Proc. Natl. Acad. Sci. USA 2004, 101, 16756–16761. [Google Scholar] [CrossRef] [Green Version]
- Krantz, B.A.; Melnyk, R.A.; Zhang, S.; Juris, S.J.; Lacy, D.B.; Wu, Z.; Finkelstein, A.; Collier, R.J. A Phenylalanine Clamp Catalyzes Protein Translocation Through the Anthrax Toxin Pore. Science 2005, 309, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Blaustein, R.; Lea, E.J.; Finkelstein, A. Voltage-Dependent Block of Anthrax Toxin Channels in Planar Phospholipid Bilayer Membranes by Symmetric Tetraalkylammonium Ions. Single-Channel Analysis. J. Gen. Physiol. 1990, 96, 921–942. [Google Scholar] [CrossRef] [Green Version]
- Blaustein, R.; Finkelstein, A. Voltage-Dependent Block of Anthrax Toxin Channels in Planar Phospholipid Bilayer Membranes by Symmetric Tetraalkylammonium Ions. Effects on Macroscopic Conductance. J. Gen. Physiol. 1990, 96, 905–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nablo, B.J.; Halverson, K.M.; Robertson, J.; Nguyen, T.L.; Panchal, R.; Gussio, R.; Bavari, S.; Krasilnikov, O.V.; Kasianowicz, J.J. Sizing the Bacillus anthracis PA63 Channel with Nonelectrolyte Poly(Ethylene Glycols). Biophys. J. 2008, 95, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Nablo, B.J.; Panchal, R.G.; Bavari, S.; Nguyen, T.L.; Gussio, R.; Ribot, W.; Friedlander, A.; Chabot, D.; Reiner, J.E.; Robertson, J.W.F.; et al. Anthrax Toxin-Induced Rupture of Artificial Lipid Bilayer Membranes. J. Chem. Phys. 2013, 139, 065101. [Google Scholar] [CrossRef] [Green Version]
- Perutz, M.F.; Rossmann, M.G.; Cullis, A.F.; Muirhead, H.; Will, G.; North, A.C.T. Structure of Hæmoglobin: A Three-Dimensional Fourier Synthesis at 5.5-Å. Resolution, Obtained by X-Ray Analysis. Nat. Cell Biol. 1960, 185, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Kendrew, J.C.; Bodo, G.; Dintzis, H.M.; Parrish, R.G.; Wyckoff, H.; Phillips, D.C. A Three-Dimensional Model of the Myoglobin Molecule Obtained by X-Ray Analysis. Nat. Cell Biol. 1958, 181, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Hobaugh, M.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J. Structure of Staphylococcal Alpha-Hemolysin, a Hep-tameric Transmembrane Pore. Science 1996, 274, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Petosa, C.; Collier, R.J.; Klimpel, K.R.; Leppla, S.H.; Liddington, R.C. Crystal Structure of the Anthrax Toxin Protective Antigen. Nat. Cell Biol. 1997, 385, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Goldie, K.N.; Abeyrathne, P.; Kebbel, F.; Chami, M.; Ringler, P.; Stahlberg, H. Cryo-Electron Microscopy of Membrane Proteins. Adv. Struct. Saf. Stud. 2014, 1117, 325–341. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Wolf, M. Cryo-Electron Microscopy for Structural Analysis of Dynamic Biological Nacromolecules. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 324–334. [Google Scholar] [CrossRef]
- Ren, G.; Quispe, J.; Leppla, S.H.; Mitra, A.K. Large-Scale Structural Changes Accompany Binding of Lethal Factor to Anthrax Protective Antigen: A Cryo-Electron Microscopic Study. Structure 2004, 12, 2059–2066. [Google Scholar] [CrossRef] [Green Version]
- Fabre, L.; Santelli, E.; Mountassif, D.; Donoghue, A.; Biswas, A.; Blunck, R.; Hanein, D.; Volkmann, N.; Liddington, R.; Rouiller, I. Structure of Anthrax Lethal Toxin Prepore Complex Suggests a Pathway for Efficient Cell Entry. J. Gen. Physiol. 2016, 148, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Gogol, E.; Engelman, D.; Zaccai, G. Neutron Diffraction Analysis of Cytochrome b5 Reconstituted in Deuterated Lipid Multilayers. Biophys. J. 1983, 43, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Torikai, N.; Yamada, N.L.; Noro, A.; Harada, M.; Kawaguchi, D.; Takano, A.; Matsushita, Y. Neutron Reflectometry on Interfacial Structures of the Thin Films of Polymer and Lipid. Polym. J. 2007, 39, 1238–1246. [Google Scholar] [CrossRef] [Green Version]
- McGillivray, D.; Valincius, G.; Heinrich, F.; Robertson, J.; Vanderah, D.J.; Febo-Ayala, W.; Ignatjev, I.; Lösche, M.; Kasianowicz, J.J. Structure of Functional Staphylococcus aureus Alpha-Hemolysin Channels in Tethered Bilayer Lipid Membranes. Biophys. J. 2009, 96, 1547–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menestrina, G. Ionic Channels Formed by Staphylococcus aureus Alpha-Toxin: Voltage-Dependent Inhibition by Divalent and Trivalent Cations. J. Membr. Biol. 1986, 90, 177–190. [Google Scholar] [CrossRef]
- Gouaux, J.E.; Braha, O.; Hobaugh, M.R.; Song, L.; Cheley, S.; Shustak, C.; Bayley, H. Subunit Stoichiometry of Staphylococcal Alpha-Hemolysin in Crystals and on Membranes: A Heptameric Transmembrane Pore. Proc. Natl. Acad. Sci. USA 1994, 91, 12828–12831. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Tao, C.; Chien, M.; Hellner, B.; Balijepalli, A.; Robertson, J.; Li, Z.; Russo, J.J.; Reiner, J.; Kasianowicz, J.J.; et al. PEG-Labeled Nucleotides and Nanopore Detection for Single Molecule DNA Sequencing by Synthesis. Sci. Rep. 2012, 2, 684. [Google Scholar] [CrossRef] [Green Version]
- Reiner, J.E.; Robertson, J.W.F.; Burden, D.L.; Burden, L.K.; Balijepalli, A.; Kasianowicz, J.J. Temperature Sculpting in Yoctoliter Volumes. J. Am. Chem. Soc. 2013, 135, 3087–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiner, J.; Balijepalli, A.; Robertson, J.; Drown, B.; Burden, D.L.; Kasianowicz, J.J. The Effects of Diffusion on an Exonuclease/Nanopore-Based DNA Sequencing Engine. J. Chem. Phys. 2012, 137, 214903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, C.; Kumar, S.; Porel, M.; Chien, M.; Bibillo, A.; Stranges, P.B.; Dorwart, M.; Tao, C.; Li, Z.; Guo, W.; et al. Real-Time Single-Molecule Electronic DNA Sequencing by Synthesis Using Polymer-Tagged Nucleotides on a Nanopore Array. Proc. Natl. Acad. Sci. USA 2016, 113, 5233–5238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Kasianowicz, J.J.; Robertson, J.; Poster, D.L.; Ettedgui, J. A Comparison of Ion Channel Current Blockades Caused by Individual Poly(ethylene glycol) Molecules and Polyoxometalate Nanoclusters. Eur. Phys. J. E 2019, 42, 83. [Google Scholar] [CrossRef] [PubMed]
- Ettedgui, J.; Kasianowicz, J.J.; Balijepalli, A. Single Molecule Discrimination of Heteropolytungstates and Their Isomers in Solution with a Nanometer-Scale Pore. J. Am. Chem. Soc. 2016, 138, 7228–7231. [Google Scholar] [CrossRef]
- Robertson, J.W.F.; Rodrigues, C.G.; Stanford, V.M.; Rubinson, K.A.; Krasilnikov, O.V.; Kasianowicz, J.J. Single-Molecule Mass Spectrometry in Solution Using a Solitary Nanopore. Proc. Natl. Acad. Sci. USA 2007, 104, 8207–8211. [Google Scholar] [CrossRef] [Green Version]
- Reiner, J.E.; Kasianowicz, J.J.; Nablo, B.J.; Robertson, J.W.F. Theory for Polymer Analysis Using Nanopore-Based Single-Molecule Mass Spectrometry. Proc. Natl. Acad. Sci. USA 2010, 107, 12080–12085. [Google Scholar] [CrossRef] [Green Version]
- Reiner, J.E.; Balijepalli, A.; Robertson, J.W.F.; Campbell, J.; Suehle, J.; Kasianowicz, J.J. Disease Detection and Management via Single Nanopore-Based Sensors. Chem. Rev. 2012, 112, 6431–6451. [Google Scholar] [CrossRef]
- Bezrukov, S.M.; Kasianowicz, J.J. Current Noise Reveals Protonation Kinetics and Number of Ionizable Sites in an Open Protein Ion Channel. Phys. Rev. Lett. 1993, 70, 2352–2355. [Google Scholar] [CrossRef] [PubMed]
- Kasianowicz, J.J.; Bezrukov, S.M. Protonation Dynamics of the Alpha-Toxin Ion Channel from Spectral Analysis of PH-Dependent Current Fluctuations. Biophys. J. 1995, 69, 94–105. [Google Scholar] [CrossRef] [Green Version]
- Cornell, B.A.; Braach-Maksvytis, V.L.B.; King, L.G.; Osman, P.D.J.; Raguse, B.; Wieczorek, L.; Pace, R.J. A Biosensor that Uses Ion-Channel Switches. Nat. Cell Biol. 1997, 387, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Knoll, W. Interfaces and Thin Films as Seen by Bound Electromagnetic Waves. Annu. Rev. Phys. Chem. 1998, 49, 569–638. [Google Scholar] [CrossRef] [Green Version]
- Valincius, G.; Mickevicius, M. Chapter Two—Tethered Phospholipid Bilayer Membranes: An Interpretation of the Electrochemical Impedance Response. In Advances in Planar Lipid Bilayers and Liposomes; Iglič, A., Kulkarni, C.V., Rappolt, M.B.T.-A., Eds.; Academic Press: New York, NY, USA, 2015; Volume 21, pp. 27–61. [Google Scholar] [CrossRef]
- Jiang, J.; Pentelute, B.L.; Collier, R.J.; Zhou, Z.H. Atomic Structure of Anthrax Protective Antigen Pore Elucidates Toxin Translocation. Nat. Cell Biol. 2015, 521, 545–549. [Google Scholar] [CrossRef]
- Benson, E.L.; Huynh, P.D.; Finkelstein, A.A.; Collier, R.J. Identification of Residues Lining the Anthrax Protective Antigen Channel. Biochemistry 1998, 37, 3941–3948. [Google Scholar] [CrossRef]
- Akabas, M.H.; Stauffer, D.A.; Xu, M.; Karlin, A. Acetylcholine Receptor Channel Structure Probed in Cysteine-Substitution Mutants. Science 1992, 258, 307–310. [Google Scholar] [CrossRef]
- Zaccai, G.; Jacrot, B. Small Angle Neutron Scattering. Annu. Rev. Biophys. Bioeng. 1983, 12, 139–158. [Google Scholar] [CrossRef] [Green Version]
- Jacrot, B. The Study of Biological Structures by Neutron Scattering from Solution. Rep. Prog. Phys. 1976, 39, 911–953. [Google Scholar] [CrossRef]
- Schoenborn, B.P. Neutrons in Biology; Springer: New York, NY, USA, 1984. [Google Scholar]
- Svergun, D.; Koch, M.H.J. Small-Angle Scattering Studies of Biological Macromolecules in Solution. Rep. Prog. Phys. 2003, 66, 1735–1782. [Google Scholar] [CrossRef]
- Ostanevich, Y.M.; Serdyuk, I.N. Neutron-Diffraction Studies of the Structure of Biological Macromolecules. Sov. Phys. Uspekhi 1982, 25, 323–339. [Google Scholar] [CrossRef]
- Feld, G.K.; Kintzer, A.F.; I Tang, I.; Thoren, K.L.; Krantz, B.A. Domain Flexibility Modulates the Heterogeneous Assembly Mechanism of Anthrax Toxin Protective Antigen. J. Mol. Biol. 2012, 415, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, H.; Polikarpov, I.; Craievich, A.F. Average Protein Density is a Molecular-Weight-Dependent Function. Protein Sci. 2009, 13, 2825–2828. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.J.; Hobbie, A.E.K.; Becker, M.L. Small-Angle Neutron Scattering from Labeled Single-Wall Carbon Nanotubes. Macromolecules 2006, 39, 2637–2642. [Google Scholar] [CrossRef]
- Schaefer, D.W.; Martin, J.E.; Wiltzius, P.; Cannell, D.S. Fractal Geometry of Colloidal Aggregates. Phys. Rev. Lett. 1984, 52, 2371–2374. [Google Scholar] [CrossRef]
- Thoren, K.L.; Krantz, B.A. The Unfolding Story of Anthrax Toxin Translocation. Mol. Microbiol. 2011, 80, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.; Holden, M.A.; Pentelute, B.L.; Collier, R.J. Ultrasensitive Detection of Protein Translocated Through Toxin Pores in Droplet-Interface Bilayers. Proc. Natl. Acad. Sci. USA 2011, 108, 16577–16581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kintzer, A.F.; Sterling, H.J.; Tang, I.I.; Williams, E.R.; Krantz, B.A. Anthrax Toxin Receptor Drives Protective Antigen Oligomerization and Stabilizes the Heptameric and Octameric Oligomer by a Similar Mechanism. PLoS ONE 2010, 5, e13888. [Google Scholar] [CrossRef] [Green Version]
- Feld, G.K.; Thoren, K.L.; Kintzer, A.F.; Sterling, H.J.; I Tang, I.; Greenberg, S.G.; Williams, E.R.; A Krantz, B. Structural Basis for the Unfolding of Anthrax Lethal Factor by Protective Antigen Oligomers. Nat. Struct. Mol. Biol. 2010, 17, 1383–1390. [Google Scholar] [CrossRef]
- Mogridge, J.; Cunningham, A.K.; Collier, R.J. Stoichiometry of Anthrax Toxin Complexes. Biochemistry 2002, 41, 1079–1082. [Google Scholar] [CrossRef]
- Blaustein, R.O.; Koehler, T.M.; Collier, R.J.; Finkelstein, A. Anthrax Toxin: Channel-Forming Activity of Protective Antigen in Planar Phospholipid Bilayers. Proc. Natl. Acad. Sci. USA 1989, 86, 2209–2213. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Andreoli, T.E.; Troutman, S.L. An Analysis of Unstirred Layers in Series with “Tight” and “Porous” Lipid Bilayer Membranes. J. Gen. Physiol. 1971, 57, 464–478. [Google Scholar] [CrossRef] [Green Version]
- Gutknecht, J.; Tosteson, D.C. Diffusion of Weak Acids across Lipid Bilayer Membranes: Effects of Chemical Reactions in the Unstirred Layers. Science 1973, 182, 1258–1261. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, S.G.; Dilger, J. Transport of Protons Across Membranes by Weak Acids. Physiol. Rev. 1980, 60, 825–863. [Google Scholar] [CrossRef]
- Pohl, P.; Saparov, S.M.; Antonenko, Y.N. The Size of the Unstirred Layer as a Function of the Solute Diffusion Coefficient. Biophys. J. 1998, 75, 1403–1409. [Google Scholar] [CrossRef] [Green Version]
- Pedley, T.J. Calculation of Unstirred Layer Thickness in Membrane Transport Experiments: A Survey. Q. Rev. Biophys. 1983, 16, 115–150. [Google Scholar] [CrossRef] [PubMed]
- Montal, M.; Mueller, P. Formation of Bimolecular Membranes from Lipid Monolayers and a Study of Their Electrical Properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [Green Version]
- Glinka, C.J.; Barker, J.G.; Hammouda, B.; Krueger, S.; Moyer, J.J.; Orts, W.J. The 30 m Small-Angle Neutron Scattering In-struments at the National Institute of Standards and Technology. J. Appl. Cryst. 1998, 31, 430–445. [Google Scholar] [CrossRef]
- Rubinson, K.A. Practical Corrections for p(H,D) Measurements in Mixed H2O/D2O Biological Buffers. Anal. Methods 2017, 9, 2744–2750. [Google Scholar] [CrossRef]
- Kline, S.R. Reduction and Analysis of SANS and USANS Data Using IGOR Pro. J. Appl. Crystallogr. 2006, 39, 895–900. [Google Scholar] [CrossRef]










| Sample/Model | Depth (Å) | Width (Å) | Length (Å) | Power Law Slope |
|---|---|---|---|---|
| pD 7.8/Right parallelepiped | 18 ± 4 | 63 ± 3 | 71 ± 4 | 2.64 ± 0.04 |
| pD 4.9/Power function | - - b | - - | - - | 2.255 ± 0.0004 |
| Model | Hole Radius (Å) | Outside Radius (Å) | Length (Å) |
|---|---|---|---|
| Homogeneous cylinder | - - a | 74 ±1 | 63 ± 3 |
| Homogeneous cylinder w/axial cylindrical hole | 19 ± 2 | 67 ± 1 | 71 ± 3 |
| Figure 5A, cryo-EM, PA637 complexed with 3 LF molecules [27] | 44 | 130 | - - |
| Figure 5B, cryo-EM, PA637 complexed with 3 LF molecules [28] | 19 | 88 | 85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michelman-Ribeiro, A.; Rubinson, K.A.; Silin, V.; Kasianowicz, J.J. Solution Structures of Bacillus anthracis Protective Antigen Proteins Using Small Angle Neutron Scattering and Protective Antigen 63 Ion Channel Formation Kinetics. Toxins 2021, 13, 888. https://doi.org/10.3390/toxins13120888
Michelman-Ribeiro A, Rubinson KA, Silin V, Kasianowicz JJ. Solution Structures of Bacillus anthracis Protective Antigen Proteins Using Small Angle Neutron Scattering and Protective Antigen 63 Ion Channel Formation Kinetics. Toxins. 2021; 13(12):888. https://doi.org/10.3390/toxins13120888
Chicago/Turabian StyleMichelman-Ribeiro, Ariel, Kenneth A. Rubinson, Vitalii Silin, and John J. Kasianowicz. 2021. "Solution Structures of Bacillus anthracis Protective Antigen Proteins Using Small Angle Neutron Scattering and Protective Antigen 63 Ion Channel Formation Kinetics" Toxins 13, no. 12: 888. https://doi.org/10.3390/toxins13120888
APA StyleMichelman-Ribeiro, A., Rubinson, K. A., Silin, V., & Kasianowicz, J. J. (2021). Solution Structures of Bacillus anthracis Protective Antigen Proteins Using Small Angle Neutron Scattering and Protective Antigen 63 Ion Channel Formation Kinetics. Toxins, 13(12), 888. https://doi.org/10.3390/toxins13120888