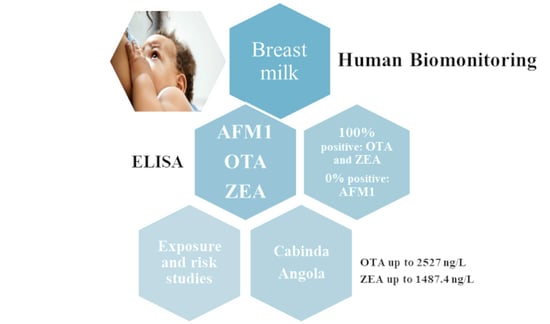
Mycotoxins Exposure in Cabinda, Angola—A Pilot Biomonitoring Survey of Breastmilk
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sociodemographic Data
2.2. Mycotoxin Contamination in Breast Milk
2.2.1. Aflatoxin M1
2.2.2. Zearalenone
2.2.3. Ochratoxin A
2.3. Exposure and Risk Assessment
2.3.1. Aflatoxin M1
2.3.2. Zearalenone
2.3.3. Ochratoxin A
3. Conclusions
4. Materials and Methods
4.1. Sampling
4.2. Sociodemographic Data and Food Consumption
4.3. Quantification of Mycotoxins
4.4. Exposure and Risk Assessment
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Braun, D.; Ezekiel, C.N.; Turner, P.C.; Degen, G.H.; Marko, D. Biomonitoring of Mycotoxins in Human Breast Milk: Current State and Future Perspectives. Chem. Res. Toxicol. 2016, 29, 1087–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2018. Food Chem. Toxicol. 2019, 129, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherkani-Hassani, A.; Mojemmi, B.; Mouane, N. Occurrence and levels of mycotoxins and their metabolites in human breast milk associated to dietary habits and other factors: A systematic literature review, 1984–2015. Trends Food Sci. Technol. 2016, 50, 56–69. [Google Scholar] [CrossRef]
- Benkerroum, N. Aflatoxins: Producing-molds, structure, health issues and incidence in southeast asian and sub-saharan african countries. Int. J. Environ. Res. Public Health 2020, 17, 1215. [Google Scholar] [CrossRef] [Green Version]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Chemical agents and related occupations. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 9.
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer—IARC Volume 56. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1993.
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Bogalho, F.; Duarte, S.; Cardoso, M.; Almeida, A.; Cabeças, R.; Lino, C.; Pena, A. Exposure assessment of Portuguese infants to Aflatoxin M1 in breast milk and maternal social-demographical and food consumption determinants. Food Control 2018, 90, 140–145. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1881/2006. Comission Regulation EC No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Huntley, B.J.; Russo, V.; Lages, F.; Almeida, N. Angola, um perfil: Fisiografia, clima e padrões de biodiversidade. In Biodiversidade de Angola, Ciência e Conservação: Uma Síntese Moderna; Arte e Ciência: Porto, Portugal, 2019; pp. 39–73. ISBN 978-989-54126-2-4. [Google Scholar]
- Pacheco, F.; Carvalho, M.L.D.S.; Henriques, P.D. Contribuição para o debate sobre a sustentabilidade da agricultura angolana. In Economia, Sociologia, Ambiente e Desenvolvimento Rural—Actas do 2º Encontro Luso-Angolano na Universidade Metodista de Angola; Universidade de Évora: Évora, Portugal, 2013; pp. 311–343. ISBN 978-989-8550-20-0. [Google Scholar]
- Fakhri, Y.; Rahmani, J.; Oliveira, C.A.F.; Franco, L.T.; Corassin, C.H.; Saba, S.; Rafique, J.; Mousavi Khaneghah, A. Aflatoxin M1 in human breast milk: A global systematic review, meta-analysis, and risk assessment study (Monte Carlo simulation). Trends Food Sci. Technol. 2019, 88, 333–342. [Google Scholar] [CrossRef]
- Hernández, M.; Juan-García, A.; Moltó, J.C.; Mañes, J.; Juan, C. Evaluation of mycotoxins in infant breast milk and infant food, reviewing the literature data. Toxins 2021, 13, 535. [Google Scholar] [CrossRef]
- Ghiasain, S.A.; Maghsood, A.H. Infants’ exposure to aflatoxin M1 from Mother’s breast milk in Iran. Iran. J. Public Health 2012, 41, 119. [Google Scholar]
- Ishikawa, A.T.; Takabayashi-Yamashita, C.R.; Ono, E.Y.S.; Bagatin, A.K.; Rigobello, F.F.; Kawamura, O.; Hirooka, E.Y.; Itano, E.N. Exposure assessment of infants to aflatoxin M1 through consumption of breast milk and infant powdered milk in Brazil. Toxins 2016, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Iha, M.H.; Barbosa, C.B.; Heck, A.R.; Trucksess, M.W. Aflatoxin M1 and ochratoxin A in human milk in Ribeirão Preto-SP, Brazil. Food Control 2014, 40, 310–313. [Google Scholar] [CrossRef] [Green Version]
- Ekeanyanwu, C.L.; Alisi, C.S.; Ekeanyanwu, R.C. Levels of Aflatoxin M1 and selected heavy metals (Pb, Cd, Cr, Cu, Zn, Fe, As, and Hg) in the breast milk of lactating mothers in South Eastern, Nigeria. Food Control 2020, 112, 107150. [Google Scholar] [CrossRef]
- Elaridi, J.; Bassil, M.; Kharma, J.A.; Daou, F.; Hassan, H.F. Analysis of aflatoxin M1 in breast milk and its association with nutritional and socioeconomic status of lactating mothers in Lebanon. J. Food Prot. 2017, 80, 1737–1741. [Google Scholar] [CrossRef]
- Azarikia, M.; Mahdavi, R.; Nikniaz, L. Occurrence and dietary factors associated with the presence of aflatoxin B1 and M1 in breast milk of nursing mothers in Iran. Food Control 2018, 86, 207–213. [Google Scholar] [CrossRef]
- Diaz, G.J.; Sánchez, M.P. Determination of aflatoxin M1 in breast milk as a biomarker of maternal and infant exposure in Colombia. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Jafari, T.; Fallah, A.A.; Kheiri, S.; Fadaei, A.; Amini, S.A. Aflatoxin M1 in human breast milk in Shahrekord, Iran and association with dietary factors. Food Addit. Contam. Part B Surveill. 2017, 10, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Memiş, E.Y.; Yalçın, S.S.; Yalçın, S. Mycotoxin carry-over in breast milk and weight of infant in exclusively-breastfed infants. Arch. Environ. Occup. Health 2021, 76, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Samiee, F.; Kharazi, A.; Elaridi, J.; Taravati Javad, M.; Leili, M. An assessment of the occurrence and nutritional factors associated with aflatoxin M1, ochratoxin A, and zearalenone in the breast milk of nursing mothers in Hamadan, Iran. Toxicon 2020, 187, 209–213. [Google Scholar] [CrossRef]
- Ortiz, J.; Jacxsens, L.; Astudillo, G.; Ballesteros, A.; Donoso, S.; Huybregts, L.; De Meulenaer, B. Multiple mycotoxin exposure of infants and young children via breastfeeding and complementary/weaning foods consumption in Ecuadorian highlands. Food Chem. Toxicol. 2018, 118, 541–548. [Google Scholar] [CrossRef]
- Karayağiz Muslu, G.; Özdemir, M. Occurrence of and Factors Associated With the Presence of Aflatoxin M1 in Breast Milk of Mothers in Fethiye, Turkey. Biol. Res. Nurs. 2020, 22, 362–368. [Google Scholar] [CrossRef]
- Braun, D.; Ezekiel, C.N.; Abia, W.A.; Wisgrill, L.; Degen, G.H.; Turner, P.C.; Marko, D.; Warth, B. Monitoring Early Life Mycotoxin Exposures via LC-MS/MS Breast Milk Analysis. Anal. Chem. 2018, 90, 14569–14577. [Google Scholar] [CrossRef] [Green Version]
- Kunter, İ.; Hürer, N.; Gülcan, H.O.; Öztürk, B.; Doğan, İ.; Şahin, G. Assessment of Aflatoxin M1 and Heavy Metal Levels in Mothers Breast Milk in Famagusta, Cyprus. Biol. Trace Elem. Res. 2017, 175, 42–49. [Google Scholar] [CrossRef]
- Cantú-Cornelio, F.; Aguilar-Toalá, J.E.; de León-Rodríguez, C.I.; Esparza-Romero, J.; Vallejo-Cordoba, B.; González-Córdova, A.F.; García, H.S.; Hernández-Mendoza, A. Occurrence and factors associated with the presence of aflatoxin M1 in breast milk samples of nursing mothers in central Mexico. Food Control 2016, 62, 16–22. [Google Scholar] [CrossRef]
- Omar, S.S. Incidence of aflatoxin M1 in human and animal milk in jordan. J. Toxicol. Environ. Health Part A Curr. Issues 2012, 75, 1404–1409. [Google Scholar] [CrossRef]
- El-Tras, W.F.; El-Kady, N.N.; Tayel, A.A. Infants exposure to aflatoxin M 1 as a novel foodborne zoonosis. Food Chem. Toxicol. 2011, 49, 2816–2819. [Google Scholar] [CrossRef] [PubMed]
- Massart, F.; Micillo, F.; Rivezzi, G.; Perrone, L.; Baggiani, A.; Miccoli, M.; Meucci, V. Zearalenone screening of human breast milk from the Naples area. Toxicol. Environ. Chem. 2016, 98, 128–136. [Google Scholar] [CrossRef]
- Dinleyici, M.; Aydemir, O.; Yildirim, G.K.; Kaya, T.B.; Carman, K.B. Human mature milk zearalenone and deoxynivalenol levels in Turkey. Neuroendocrinol. Lett. 2018, 39, 325–330. [Google Scholar] [PubMed]
- Valitutti, F.; De Santis, B.; Trovato, C.M.; Montuori, M.; Gatti, S.; Oliva, S.; Brera, C.; Catassi, C. Assessment of mycotoxin exposure in breastfeeding mothers with celiac disease. Nutrients 2018, 10, 336. [Google Scholar] [CrossRef] [Green Version]
- Braun, D.; Schernhammer, E.; Marko, D.; Warth, B. Longitudinal assessment of mycotoxin co-exposures in exclusively breastfed infants. Environ. Int. 2020, 142, 105845. [Google Scholar] [CrossRef]
- Rubert, J.; León, N.; Sáez, C.; Martins, C.P.B.; Godula, M.; Yusà, V.; Mañes, J.; Soriano, J.M.; Soler, C. Evaluation of mycotoxins and their metabolites in human breast milk using liquid chromatography coupled to high resolution mass spectrometry. Anal. Chim. Acta 2014, 820, 39–46. [Google Scholar] [CrossRef]
- Muñoz, K.; Blaszkewicz, M.; Campos, V.; Vega, M.; Degen, G.H. Exposure of infants to ochratoxin A with breast milk. Arch. Toxicol. 2014, 88, 837–846. [Google Scholar] [CrossRef]
- Kamali, A.; Mehni, S.; Kamali, M.; Sarvtin, M.T. Detection of ochratoxin A in human breast milk in Jiroft city, south of Iran. Curr. Med. Mycol. 2017, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Biasucci, G.; Calabrese, G.; Di Giuseppe, R.; Carrara, G.; Colombo, F.; Mandelli, B.; Maj, M.; Bertuzzi, T.; Pietri, A.; Rossi, F. The presence of ochratoxin A in cord serum and in human milk and its correspondence with maternal dietary habits. Eur. J. Nutr. 2011, 50, 211–218. [Google Scholar] [CrossRef]
- Hassan, A.M.; Sheashaa, H.A.; Fattah, M.F.A.; Ibrahim, A.Z.; Gaber, O.A.; Sobh, M.A. Study of ochratoxin A as an environmental risk that causes renal injury in breast-fed Egyptian infants. Pediatr. Nephrol. 2006, 21, 102–105. [Google Scholar] [CrossRef]
- Afshar, P.; Shokrzadeh, M.; Kalhori, S.; Babaee, Z.; Saeedi Saravi, S.S. Occurrence of Ochratoxin A and Aflatoxin M1 in human breast milk in Sari, Iran. Food Control 2013, 31, 525–529. [Google Scholar] [CrossRef]
- Gimbi, M. Biomonitorização de Ocratoxina a em Leite Materno: Avaliação da Exposição dos Lactentes. Ph.D. Thesis, Universidade de Coimbra, Coimbra, Portugal, 2019. [Google Scholar]
- Dehghan, P.; Pakshir, K.; Rafiei, H.; Chadeganipour, M.; Akbari, M. Prevalence of ochratoxin A in human milk in the Khorrambid Town, Fars Province, south of Iran. Jundishapur J. Microbiol. 2014, 7, e11220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; Nielsen, E.; et al. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, e06113. [Google Scholar] [CrossRef]
- Assunção, R.; Martins, C.; Vasco, E.; Jager, A.; Oliveira, C.; Cunha, S.C.; Fernandes, J.O.; Nunes, B.; Loureiro, S.; Alvito, P. Portuguese children dietary exposure to multiple mycotoxins—An overview of risk assessment under MYCOMIX project. Food Chem. Toxicol. 2018, 118, 399–408. [Google Scholar] [CrossRef]
- Raiola, A.; Tenore, G.C.; Manyes, L.; Meca, G.; Ritieni, A. Risk analysis of main mycotoxins occurring in food for children: An overview. Food Chem. Toxicol. 2015, 84, 169–180. [Google Scholar] [CrossRef]
- Watson, S.; Chen, G.; Sylla, A.; Routledge, M.N.; Gong, Y.Y. Dietary exposure to aflatoxin and micronutrient status among young children from Guinea. Mol. Nutr. Food Res. 2016, 60, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Djamen, C. Safety of breast milk vis-à-vis common infant formula and complementary foods from mycotoxin perspective. Recent Adv. Food Sci. 2018, 1, 23–31. [Google Scholar]
- Degen, G.H.; Partosch, F.; Muñoz, K.; Gundert-Remy, U. Daily uptake of mycotoxins—TDI might not be protective for nursed infants. Toxicol. Lett. 2017, 277, 69–75. [Google Scholar] [CrossRef]
- World Health Organization & Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Contaminants in Food: Eighty-Third Report of the JOINT FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA J. 2017, 15, e04849. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki. Bull. World Health Organ. 2001, 79, 373. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Taipei; World Medical Association: Ferney-Voltaire, France, 2016. [Google Scholar]
- Diário da República, n.º 2/2001, Série I-A de 2001-01-03 (2001) pages 14–36. Available online: https://dre.pt/web/guest/pesquisa/-/search/235127/details/maximized (accessed on 2 January 2022).
- EFSA. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- EFSA. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to ochratoxin a in food. EFSA J. 2006, 4, 365. [Google Scholar] [CrossRef]
| Variable | Frequency (%) | Mean ± SD | Range (Years) | Average [AFM1] ± SD [Min–Max] (ng/L) | Average [ZEN] ±SD [Min–Max] (ng/L) | Average [OTA] ±SD [Min–Max] (ng/L) |
|---|---|---|---|---|---|---|
| Mother age | 26.7 ± 7.1 years | 18–40 years | <LOD | |||
| 18–25 years | 17/37 (45%) | 377 ± 219 [72.5–1077.9] | 714.5 ± 370.3 [312.9–1791.6] | |||
| 26–33 years | 12/37 (32%) | 436 ± 360 [185.2–1487.4] | 678.0 ± 363.2 [290.6–1419.4] | |||
| ≥34 years | 7/37 (19%) | 310 ± 124 [138.2–476.6] | 777.3 ± 832.9 [285.1–2527.1] | |||
| No answer | 1/37 (3%) | 268.3 | 181.1 | |||
| Profession | n.a. | n.a. | <LOD | |||
| Student | 17/37 (46%) | 402.9 ± 300.9 [166.2–1487.4] | 591.4 ± 227.7 [313.0–1039.3] | |||
| Domestic | 14/37 (38%) | 368.9 ± 255.2 [72.5–1077.9] | 844.2 ± 688.1 [181.1–2527.1] | |||
| Teacher | 3/37 (8%) | 383.0 ± 148.2 [212.2–476.6] | 531.5 ± 367.3 [285.1–953.7] | |||
| Operator | 1/37 (2%) | 237.2 | 1171.1 | |||
| Tourism technician | 1/37 (3%) | 317.1 | 815.5 | |||
| No answer | 1/37 (3%) | 369.7 | 452.1 | |||
| Education level | n.a. | n.a. | <LOD | |||
| Elementary education | 24/37 (65%) | 408.0 ± 301.7 [72.5–1487.4] | 664.9 ± 392.8 [285.1–1791.6] | |||
| High school | 10/37 (27%) | 357.1 ± 141.5 (212.2–593.7] | 642.3 ± 315.5 [313.0–1171.1] | |||
| Higher education | 1/37 (3%) | 317.1 | 815.5 | |||
| No answer | 2/37 (5%) | 203.3 ± 92.0 [138.2–268.3] | 1354.1 ± 1658.9 [181.1–2527.1] | |||
| Variable | Frequency (%) | Mean ± SD | Range (Years) | Average [AFM1] ±SD [Min–Max] (ng/L) | Average 1 [ZEN] ±SD [Min–Max] (ng/L) | Average 1 [OTA] ±SD [Min–Max] (ng/L) |
| Number of children | 3.0 ± 1.9 children | 1–9 children | <LOD | |||
| 1 | 10/37 (27%) | 612.0 ± 318.0 [166.2–1077.9] | 678.4 ± 215.3 [351.0–1123.2] | |||
| 2 | 5/37 (14%) | 325.0 ± 148.0 [212.2–575.1] | 573.0 ± 293.6 [313.0–953.7] | |||
| 3 | 9/37 (24%) | 459.8 ± 413.9 [72.5–1487.4] | 712.3 ± 485.7 [285.1–1791.6] | |||
| ≥4 | 12/37 (35%) | 300.2 ± 96.7 [138.2–476.6] | 796.2 ± 681.8 [181.1–2527.1] | |||
| No answer | 1/37 (3%) | 600.2 | 290.6 | |||
| Infant age | 6.0 ± 3.5 months | 1–18 months | <LOD | |||
| 1–3 months | 8/37 (22%) | 372.1 ± 305.5 [138.2–1077.9] | 800.5 ± 762.1 [181.1–2527.1] | |||
| 4–6 months | 10/37 (27%) | 323.1 ± 164.0 [72.5–600.2] | 775.2 ± 540.5 [290.6–1791.6] | |||
| 7–10 months | 17/37 (46%) | 416.2 ± 297.3 [186.93–1487.4] | 579.9 ± 222.7 [297.6–967.4] | |||
| ≥12 months | 2/37 (5%) | 401.2 ± 83.5 [342.2–460.2] | 945.8 ± 368.8 [685.1–1206.6] | |||
| Weight of infants 2 | 7.5 ± 1.8 kg | 3.5–11.4 kg | <LOD | |||
| 3.5–6.9 kg | 10/37 (27%) | 299.0 ± 95.0 [138.2–460.2] | 886.3 ± 708.0 [181.1–2527.1] | |||
| 7–9 kg | 21/37 (57%) | 382.0 ± 216.0 [72.5–1077.9] | 679.2 ± 374.3 [290.6–1791.6] | |||
| ≥9.1 kg | 5/37 (14%) | 598.0 ± 501.0 [314.9–147.4] | 492.7 ± 155.8 [351.0–685.1] | |||
| No answer | 1/37 (2.7%) | 242.52 | 316.7 |
| Country (Year) | Frequency of Contamination (%) | AFM1 Concentration (ng/L) | Analytical Method (LOD ng/L) | Reference | |
|---|---|---|---|---|---|
| Mean ± SD | Range | ||||
| Angola (2018–2019) | 0/37 (0%) | n.a. | <5.0 | ELISA (5.0) | Present study |
| Turkey (2017/2018) | 78//79 (98,7%) | 3.0 (median) | 2.59–3.8 | ELISA (2.0) | [26] |
| Iran (2019) | 47/90 (52.2%) 17/90 (18.9%) | 6.0 ± 1.5 4.4 ± 1.2 | 5.16–13.5 6.05–12.1 | ELISA (5.0) HPLC (n.a.) | [27] |
| Nigeria (2019) | 225/225 (100%) | 4.0 ± 1.1 | 2.33–7.1 | HPLC (n.a.) | [21] |
| Ecuator (2012–2013) | 10/78 (13%) | 216 ± 116 | 53–458 | IAC/HPLC-FD (33/23) | [28] |
| Turkey (2017) | 53/100 (53%) | 6.4 | 5.10–8.3 | ELISA (5.0) | [29] |
| Iran (2016) | 39/250 (15.6%) | 21.0 ± 1.0 | 11.07–39.3 | ELISA (2.3) | [25] |
| Nigeria | 1/75 (1.3%) | <LOQ (87) | n.a. | QuEChERS/LC-MS/MS (43.0) | [30] |
| Portugal (2015–2016) | 22/67 (32.8%) | 7.4 ± 1.9 | 5.1 ± 10.6 | ELISA (5.0) | [12] |
| Lebanon (2015–2016) | 104/111 (93.8%) | 4.3 ± 1.8 | 0.22–7.9 | ELISA (0.2) | [21] |
| Iran (2015) | 88/88 (100%) | 3.2 | 0.1–13.6 | ELISA (0.04) | [23] |
| Cyprus (2015) | 40/50 (80%) | 7.8 ± 1.7 | 5.36–28.4 | ELISA (5.0) | [31] |
| Mexico (2014) | 100/112 (89%) | 10.4 | 3.01–34.2 | ELISA (0.92) | [32] |
| Brazil (2013) | 5/94 (5.3%) | 18 ± 5 | 13–25 | HPLC (4.0) | [19] |
| Colombia (2013) | 45/50 (90%) | 5.2 | 0.9–18.5 | HPLC (0.6) | [24] |
| Brazil (2011–2012) | 2/100 (2%) | 0.55 | 0.3–0.8 | LC (0.3) | [20] |
| Jordan (2011) | 80/80 (100%) | 67.8 ± 4.6 | 9.7–137.2 | ELISA (5.0) | [33] |
| Egypt (2010) | 87/125 (69.6%) | 74.4 ± 7.1 | 7.3–328.6 | ELISA (5.0) | [34] |
| Country (Year) | Frequency of Contamination (%) | ZEN Concentration (ng/L) | Analytical Method (LOD/LOQ ng/L) | Reference | |
|---|---|---|---|---|---|
| Mean ± SD | Range | ||||
| Angola (2018/2019) | 37/37 (100%) | 380.7 ± 256.7 | 72.5–1487.4 | ELISA (60) | Present study |
| Austria (2015/2016) | 0/87 * | n.a. | n.a. | LC-MS/MS (-/32) | [38] |
| Iran (2019) | 0/90 (0%) 0/90 (0%) | n.a. n.a. | n.a. n.a. | ELISA (5) HPLC (5) | [27] |
| Turkey (2017/2018) | 79/79 (100%) | 340 (median) | 230–510 | ELISA | [26] |
| Nigeria | 0/75 (0%) | n.a. | n.a. | QuEChERS/LC-MS/MS (93/190) | [30] |
| Turkey | 90/90 (100%) | 173.8 (median) | 35.7–682 | ELISA | [36] |
| Italy (2011/2013) | Mothers with celiac disease: 12/275 (4%) Control mothers: 15/178 (8%) | 2100 2700 | 2000–17,000 2000–22,000 | IAC/HPLC-FD (-/4000) | [37] |
| Italy | 47/47 (100%) | 1130 ± 340 | 260–1780 | ELISA (60) and LC-FD (20/50) | [35] |
| Spain (2012) | 13/35 (37%) | n.a. | 2100–14,300 | QuEChERS/UHPLC-HRMS | [39] |
| Country (Year) | Frequency of Contamination (%) | OTA Concentration (ng/L) | Analytical Method (LOD/LOQ ng/L) | Reference | |
|---|---|---|---|---|---|
| Mean ± SD | Range | ||||
| Angola (2018/2019) | 37/37 (100%) | 700.1 ± 475.1 | 181.1–2527.1 | ELISA (150) | Present study |
| Turkey (2017/2018) | -/79 | 340 (median) | - | ELISA | [26] |
| Iran (2019) | 0/90 (0%) 0/90 (0%) | - - | - - | ELISA (5000) HPLC (5000) | [27] |
| Portugal (2015–2019) | 41/42 (97.6%) | 305.5 ± 114 | 59.4–559.6 | ELISA (50) | [45] |
| Nigeria | 11/75 (14.7%) | <LOQ (96) | - | QuEChERS/LC-MS/MS (48) | [30] |
| Iran (2016–2017) | 14/84 (16.6%) | 1990 ± 1340 | 110–7340 | ELISA (3000) | [41] |
| Italy (2011/2013) | Mothers with celiac disease: 6/275 (2%) Control mothers: 1/178 (0.5%) | LOQ-0.123 LOQ-56 | IAC/HPLC-FD (-/34) | [37] | |
| Iran (2011) | 84/87 (96.6%) | 24.57 ± 13.6 | 1.6–60 | ELISA | [46] |
| Brazil (2011–2012) | 66/100 (66%) | 4 | LOQ-21 | IAC/HPLC-FD (0.3/0.8) | [20] |
| Chile (2008; 2010) | LLE/HPLC-FD (10/30) | [40] | |||
| Colostrum (1–6 days) | 14/17 (82%) | 86 ± 59 | LOD-186 | ||
| Transition milk (15–30 days) | 10/15 (67%) | 33 ± 27 | LOD-81 | ||
| Mature milk (2 months) | 6/7 (86%) | 27 ± 19 | LOD-52 | ||
| Mature milk (4 months) | 5/6 (83%) | 30 ± 14 | LOD-43 | ||
| Mature milk (6 months) | 5/5 (100%) | 44 ± 18 | 18–63 | ||
| Iran (2011) | 2/136 (2.72%) | 115 | 90–140 | HPLC-FD | [44] |
| Italy (2007) | 41/57 (78.8%) | 10 ± 15.6 | LOD-75.1 | IAC/HPLC-FD (0.5/1.0) | [42] |
| Breastfed < 7 kg (n = 11) | Breastfed ≥ 7 kg (n = 25) | Infants < 16 Weeks of Age (n = 8) | |||||
|---|---|---|---|---|---|---|---|
| EDI | HI | EDI | HI | EDI a | HI b | ||
| Zearalenone | Lowest level (Best-case scenario) | 20.7 | 0.1 | 10.1 | 0.1 | 35.9 | 0.43 |
| Average level (Average-case scenario) | 44.9 | 0.2 | 50 | 0.6 | 96.8 | 1.2 | |
| Highest level (Worst-case scenario) | 69 | 0.8 | 159.9 | 1.9 | 280.3 | 3.4 | |
| Ochratoxin A | Lowest level (Best-case scenario) | 27.2 | - | 37.3 | - | 47.1 | - |
| Average level (Average-case scenario) | 132.9 | - | 76.1 | - | 208.1 | - | |
| Highest level (Worst-case scenario) | 379.1 | - | 248.8 | - | 657.0 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, S.; Silva, L.J.G.; Pereira, A.M.P.T.; Gimbi, M.; Cesar, C.; Vidal, V.; Basílio, R.; Almeida, A.; Lino, C.; Pena, A. Mycotoxins Exposure in Cabinda, Angola—A Pilot Biomonitoring Survey of Breastmilk. Toxins 2022, 14, 204. https://doi.org/10.3390/toxins14030204
Duarte S, Silva LJG, Pereira AMPT, Gimbi M, Cesar C, Vidal V, Basílio R, Almeida A, Lino C, Pena A. Mycotoxins Exposure in Cabinda, Angola—A Pilot Biomonitoring Survey of Breastmilk. Toxins. 2022; 14(3):204. https://doi.org/10.3390/toxins14030204
Chicago/Turabian StyleDuarte, Sofia, Liliana J. G. Silva, André M. P. T. Pereira, Marta Gimbi, Cristiane Cesar, Vanessa Vidal, Rita Basílio, Anabela Almeida, Celeste Lino, and Angelina Pena. 2022. "Mycotoxins Exposure in Cabinda, Angola—A Pilot Biomonitoring Survey of Breastmilk" Toxins 14, no. 3: 204. https://doi.org/10.3390/toxins14030204
APA StyleDuarte, S., Silva, L. J. G., Pereira, A. M. P. T., Gimbi, M., Cesar, C., Vidal, V., Basílio, R., Almeida, A., Lino, C., & Pena, A. (2022). Mycotoxins Exposure in Cabinda, Angola—A Pilot Biomonitoring Survey of Breastmilk. Toxins, 14(3), 204. https://doi.org/10.3390/toxins14030204