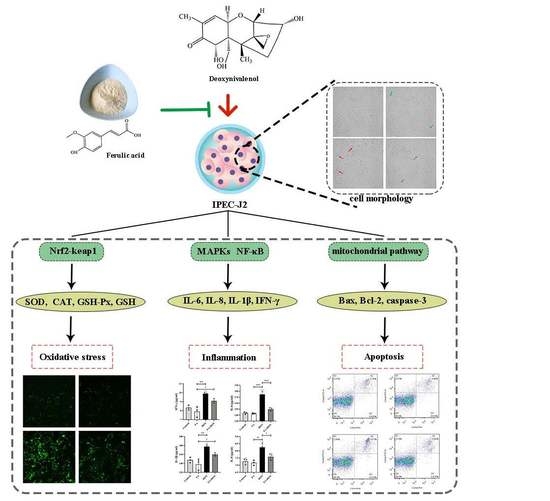
Protective Effects of Ferulic Acid on Deoxynivalenol-Induced Toxicity in IPEC-J2 Cells
Abstract
:1. Introduction
2. Results
2.1. FA and DON on Cell Viability of IPEC-J2 Cells
2.2. FA Alleviating DON-Induced IPEC-J2 Cell Cytotoxicity
2.3. FA Inhibits DON-Induced Oxidative Stress
2.3.1. Elimination of ROS by FA
2.3.2. Effect of FA on CAT, SOD, GSH-Px, and GSH
2.3.3. Activation of the Nrf2-keap1 Signaling Pathway
2.4. FA Inhibits DON-Induced Inflammation
2.4.1. FA Inhibits the Production of the Inflammatory Cytokines
2.4.2. FA Inhibits the Phosphorylation of MAPKs Pathway Related Proteins
2.4.3. FA Inhibits the Activation of the NF-κB Pathway
2.5. FA Inhibits DON-Induced Apoptosis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Treatments
4.3. Cell Viability and Cell Morphology Observation
4.4. Intracellular Reactive Oxygen Species (ROS) Concentration
4.5. Antioxidant Index Determination
4.6. Enzyme-Linked Immunosorbent Assay
4.7. Flow-Cytometric Determination of Apoptosis
4.8. Quantitative Real-Time PCR
4.9. Western Blot Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salman, M.K.; Mudalal, S. Quality control and mycotoxin levels in food in the Palestinian market. Food Addit. Contam. Part B 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Gagiu, V.; Mateescu, E.; Dobre, A.A.; Smeu, I.; Cucu, M.E.; Oprea, O.A.; Alexandru, D.; Iorga, E.; Belc, N. Deoxynivalenol Occurrence in Triticale Crops in Romania during the 2012–2014 Period with Extreme Weather Events. Toxins 2021, 13, 456. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Liu, Z.; Liu, S.; Yao, L.; Liu, Y.; Wu, Y.; Gong, Z. Natural Occurrence of Deoxynivalenol and Its Acetylated Derivatives in Chinese Maize and Wheat Collected in 2017. Toxins 2020, 12, 200. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Kuca, K.; Humpf, H.U.; Klimova, B.; Cramer, B. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during cereal-based thermal food processing: A review study. Mycotoxin Res. 2017, 33, 79–91. [Google Scholar] [CrossRef]
- Hooft, J.M.; Bureau, D.P. Deoxynivalenol: Mechanisms of action and its effects on various terrestrial and aquatic species. Food Chem. Toxicol. 2021, 157, 112616. [Google Scholar] [CrossRef]
- Vignal, C.; Djouina, M.; Pichavant, M.; Caboche, S.; Waxin, C.; Beury, D.; Hot, D.; Gower-Rousseau, C.; Body-Malapel, M. Chronic ingestion of deoxynivalenol at human dietary levels impairs intestinal homeostasis and gut microbiota in mice. Arch. Toxicol. 2018, 92, 2327–2338. [Google Scholar] [CrossRef]
- Qu, R.; Jiang, C.; Wu, W.; Pang, B.; Lei, S.; Lian, Z.; Shao, D.; Jin, M.; Shi, J. Conversion of DON to 3-epi-DON in vitro and toxicity reduction of DON in vivo by Lactobacillus rhamnosus. Food Funct. 2019, 10, 2785–2796. [Google Scholar] [CrossRef]
- Pinton, P.; Braicu, C.; Nougayrede, J.P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef] [Green Version]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef]
- Kalaiselvi, P.; Rajashree, K.; Bharathi Priya, L.; Padma, V.V. Cytoprotective effect of epigallocatechin-3-gallate against deoxynivalenol-induced toxicity through anti-oxidative and anti-inflammatory mechanisms in HT-29 cells. Food Chem. Toxicol. 2013, 56, 110–118. [Google Scholar] [CrossRef]
- Krishnaswamy, R.; Devaraj, S.N.; Padma, V.V. Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: Prevention of NF-kappaB nuclear localization and down regulation of NF-kappaB and Cyclo-Oxygenase-2 expression. Free Radic. Biol. Med. 2010, 49, 50–60. [Google Scholar] [CrossRef]
- Salah, A.; Bouaziz, C.; Amara, I.; Abid-Essefi, S.; Bacha, H. Eugenol protects against citrinin-induced cytotoxicity and oxidative damages in cultured human colorectal HCT116 cells. Environ. Sci. Pollut. Res. Int. 2019, 26, 31374–31383. [Google Scholar] [CrossRef]
- Sang, Y.; Li, W.; Zhang, G. The protective effect of resveratrol against cytotoxicity induced by mycotoxin, zearalenone. Food Funct. 2016, 7, 3703–3715. [Google Scholar] [CrossRef]
- Chaudhary, A.; Jaswal, V.S.; Choudhary, S.; Sonika; Sharma, A.; Beniwal, V.; Tuli, H.S.; Sharma, S. Ferulic Acid: A Promising Therapeutic Phytochemical and Recent Patents Advances. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 115–123. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [Green Version]
- Erseckin, V.; Mert, H.; Irak, K.; Yildirim, S.; Mert, N. Nephroprotective effect of ferulic acid on gentamicin-induced nephrotoxicity in female rats. Drug Chem. Toxicol. 2020, 45, 1–7. [Google Scholar] [CrossRef]
- Mishra, S.; Dixit, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Influence of temperature and pH on the degradation of deoxynivalenol (DON) in aqueous medium: Comparative cytotoxicity of DON and degraded product. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 121–131. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.; Fan, M.; Meng, T.; Chen, X.; Jiang, Y.; Zhu, D.; Hu, W.; Gong, J.; Feng, S.; et al. Deoxynivalenol induces apoptosis in PC12 cells via the mitochondrial pathway. Environ. Toxicol. Pharmacol. 2016, 43, 193–202. [Google Scholar] [CrossRef]
- Ji, R.; Jia, F.Y.; Chen, X.; Wang, Z.H.; Jin, W.Y.; Yang, J. Salidroside alleviates oxidative stress and apoptosis via AMPK/Nrf2 pathway in DHT-induced human granulosa cell line KGN. Arch. Biochem. Biophys. 2022, 715, 109094. [Google Scholar] [CrossRef]
- Singh, S.; Vrishni, S.; Singh, B.K.; Rahman, I.; Kakkar, P. Nrf2-ARE stress response mechanism: A control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic. Res. 2010, 44, 1267–1288. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Z.; Shen, B.; Wu, L.; Han, L.; Zhang, Q.; Feng, H. The protective effects of Shikonin on lipopolysaccharide/d-galactosamine-induced acute liver injury via inhibiting MAPK and NF-κB and activating Nrf2/HO-1 signaling pathways. RSC Adv. 2017, 7, 34846–34856. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Qin, X.; Tian, J.; Gao, X.; Wu, X.; Du, G.; Zhou, Y. Liquiritin protects PC12 cells from corticosterone-induced neurotoxicity via regulation of metabolic disorders, attenuation ERK1/2-NF-kappaB pathway, activation Nrf2-Keap1 pathway, and inhibition mitochondrial apoptosis pathway. Food Chem. Toxicol. 2020, 146, 111801. [Google Scholar] [CrossRef]
- Guon, T.E.; Chung, H.S. Moringa oleifera fruit induce apoptosis via reactive oxygen species-dependent activation of mitogen-activated protein kinases in human melanoma A2058 cells. Oncol. Lett. 2017, 14, 1703–1710. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Guo, Q.; Liang, Z.; Wang, M.; Wang, B.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Wang, J.; Ma, C.; Kang, W. Anti-inflammatory and antioxidant effects of Chaetoglobosin Vb in LPS-induced RAW264.7 cells: Achieved via the MAPK and NF-kappaB signaling pathways. Food Chem. Toxicol. 2021, 147, 111915. [Google Scholar] [CrossRef]
- Yuan, J.; Che, S.; Zhang, L.; Ruan, Z. Reparative Effects of Ethanol-Induced Intestinal Barrier Injury by Flavonoid Luteolin via MAPK/NF-kappaB/MLCK and Nrf2 Signaling Pathways. J. Agric. Food Chem. 2021, 69, 4101–4110. [Google Scholar] [CrossRef]
- Chen, X.; Gu, M.; Jin, J.; Ren, C.; Pan, Z.; Wu, Y.; Tian, N.; Wu, A.; Sun, L.; Gao, W.; et al. beta-Hydroxyisovalerylshikonin inhibits IL-1beta-induced chondrocyte inflammation via Nrf2 and retards osteoarthritis in mice. Food Funct. 2020, 11, 10219–10230. [Google Scholar] [CrossRef]
- Yin, Z.; Guo, H.; Jiang, K.; Ou, J.; Wang, M.; Huang, C.; Liu, F.; Bai, W.; Zheng, J.; Ou, S. Morin decreases acrolein-induced cell injury in normal human hepatocyte cell line LO2. J. Funct. Foods 2020, 75, 104234. [Google Scholar] [CrossRef]
- Dey, D.K.; Chang, S.N.; Kang, S.C. The inflammation response and risk associated with aflatoxin B1 contamination was minimized by insect peptide CopA3 treatment and act towards the beneficial health outcomes. Environ. Pollut. 2021, 268, 115713. [Google Scholar] [CrossRef]
- Yu, Y.H.; Lai, Y.H.; Hsiao, F.S.; Cheng, Y.H. Effects of Deoxynivalenol and Mycotoxin Adsorbent Agents on Mitogen-Activated Protein Kinase Signaling Pathways and Inflammation-Associated Gene Expression in Porcine Intestinal Epithelial Cells. Toxins 2021, 13, 301. [Google Scholar] [CrossRef]
- Wang, X.; Fan, M.; Chu, X.; Zhang, Y.; Rahman, S.U.; Jiang, Y.; Chen, X.; Zhu, D.; Feng, S.; Li, Y.; et al. Deoxynivalenol induces toxicity and apoptosis in piglet hippocampal nerve cells via the MAPK signaling pathway. Toxicon 2018, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhao, Y.; Yang, Y.; Han, X.; Duan, J. Astragaloside IV protects against retinal iron overload toxicity through iron regulation and the inhibition of MAPKs and NF-kappaB activation. Toxicol. Appl. Pharmacol. 2021, 410, 115361. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, B.; Sun, X.; Li, Z.; Chen, Y.; Guo, Z.; Liu, H.; Li, D.; Wang, C.; Zhu, X.; et al. Protective effects of alfalfa saponins on oxidative stress-induced apoptotic cells. Food Funct. 2020, 11, 8133–8140. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chang, J.; Wang, P.; Yin, Q.; Liu, C.; Li, M.; Song, A.; Zhu, Q.; Lu, F. Effect of chlorogenic acid on alleviating inflammation and apoptosis of IPEC-J2 cells induced by deoxyniyalenol. Ecotoxicol. Environ. Saf. 2020, 205, 111376. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Yu, R.; Zhou, Q.; Liu, P.; Liu, Z.; Bian, Y. Naringenin prevents TNF-alpha-induced gut-vascular barrier disruption associated with inhibiting the NF-kappaB-mediated MLCK/p-MLC and NLRP3 pathways. Food Funct. 2021, 12, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, B.R.; Zhang, Q.; Zhao, X.H.; Wang, L. Pretreatment of IEC-6 cells with quercetin and myricetin resists the indomethacin-induced barrier dysfunction via attenuating the calcium-mediated JNK/Src activation. Food Chem. Toxicol. 2021, 147, 111896. [Google Scholar] [CrossRef]
- Deng, Y.; Qiu, M.; Wang, Y.; Wang, R.; Lu, P.; Sun, L.; Li, X.; Gooneratne, R. Protective effect of antioxidant-enriched diets on T-2-toxin-induced damage in tilapia (Oreochromis niloticus). Aquaculture 2019, 506, 341–349. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, C.; Ye, J.; Lv, Y.; Wang, L.; Chen, Z.; Jiang, Z. Protection of Porcine Intestinal-Epithelial Cells from Deoxynivalenol-Induced Damage by Resveratrol via the Nrf2 Signaling Pathway. J. Agric. Food Chem. 2019, 67, 1726–1735. [Google Scholar] [CrossRef]
- Meng, M.; Zhang, R.; Han, R.; Kong, Y.; Wang, R.; Hou, L. The polysaccharides from the Grifola frondosa fruiting body prevent lipopolysaccharide/D-galactosamine-induced acute liver injury via the miR-122-Nrf2/ARE pathways. Food Funct. 2021, 12, 1973–1982. [Google Scholar] [CrossRef]
- Chen, D.; Tavana, O.; Gu, W. ARF-NRF2: A new checkpoint for oxidative stress responses? Mol. Cell Oncol. 2018, 5, e1432256. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Shi, D.; Sun, J.; Li, H.; Zhao, M.; Sun, B. Quantification and cytoprotection by vanillin, 4-methylguaiacol and 4-ethylguaiacol against AAPH-induced abnormal oxidative stress in HepG2 cells. RSC Adv. 2018, 8, 35474–35484. [Google Scholar] [CrossRef] [Green Version]
- Long, M.; Yang, S.H.; Shi, W.; Li, P.; Guo, Y.; Guo, J.; He, J.B.; Zhang, Y. Protective effect of proanthocyanidin on mice Sertoli cell apoptosis induced by zearalenone via the Nrf2/ARE signalling pathway. Environ. Sci. Pollut. Res. Int. 2017, 24, 26724–26733. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, D.; Sun, J.; Luo, X.; Li, H.; Sun, X.; Zheng, F. Analysis of antioxidant effect of two tripeptides isolated from fermented grains (Jiupei) and the antioxidative interaction with 4-methylguaiacol, 4-ethylguaiacol, and vanillin. Food Sci. Nutr. 2019, 7, 2391–2403. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, Y.; Jiao, D.; Yang, S.; Li, L.; Li, P. Protective Effect of Astaxanthin on Ochratoxin A-Induced Kidney Injury to Mice by Regulating Oxidative Stress-Related NRF2/KEAP1 Pathway. Molecules 2020, 25, 1386. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Piao, R.; Wang, H.; Li, C.; Song, L. Orientin-mediated Nrf2/HO-1 signal alleviates H2O2-induced oxidative damage via induction of JNK and PI3K/AKT activation. Int. J. Biol. Macromol. 2018, 118, 747–755. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.; Wu, W.; Wang, J.; Xie, H.; Wu, Z. Regeneration of glutathione by alpha-lipoic acid via Nrf2/ARE signaling pathway alleviates cadmium-induced HepG2 cell toxicity. Environ. Toxicol. Pharmacol. 2017, 51, 30–37. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, R.; Yin, Z.; Sun, J.; Wang, B.; Zhao, D.; Zeng, X.A.; Li, H.; Huang, M.; Sun, B. Optimization of Jiuzao protein hydrolysis conditions and antioxidant activity in vivo of Jiuzao tetrapeptide Asp-Arg-Glu-Leu by elevating the Nrf2/Keap1-p38/PI3K-MafK signaling pathway. Food Funct. 2021, 12, 4808–4824. [Google Scholar] [CrossRef]
- Ko, W.C.; Shieh, J.M.; Wu, W.B. P38 MAPK and Nrf2 Activation Mediated Naked Gold Nanoparticle Induced Heme Oxygenase-1 Expression in Rat Aortic Vascular Smooth Muscle Cells. Arch. Med. Res. 2020, 51, 388–396. [Google Scholar] [CrossRef]
- Peng, C.; Sun, Z.; Wang, L.; Shu, Y.; He, M.; Ding, H.; Li, Y.; Wang, X.; Feng, S.; Li, J.; et al. Soybean antigen protein induces caspase-3/mitochondrion-regulated apoptosis in IPEC-J2 cells. Food Agric. Immunol. 2019, 31, 100–119. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Zhou, Y.; Lin, X.; Duan, N.; Wang, Z.; Wu, S. Deoxynivalenol-induced cell apoptosis monitoring using a cytochrome c-specific fluorescent probe based on a photoinduced electron transfer reaction. J. Hazard. Mater. 2021, 415, 125638. [Google Scholar] [CrossRef]








| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| keap1 | ATGGCGGGGCCTCTGA | CTCAGGGGCAGAAATTGGGT |
| HO-1 | GGCTGAGAATGCCGAGTTCA | GGACGCCATCACCAGCTTAAA |
| Bax | GCCCTTTTGCTTCAGGGTTTC | CAATGCGCTTGAGACACTCG |
| Bcl-2 | GATAACGGAGGCTGGGATGC | TTATGGCCCAGATAGGCACC |
| Caspase-3 | GGAATGGCATGTCGATCTGGT | ACTGTCCGTCTCAATCCCAC |
| GAPDH | ATGACCACAGTCCATGCCATC | CCTGCTTCACCACCTTCTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Yu, W.; Duan, N.; Wang, Z.; Shen, Y.; Wu, S. Protective Effects of Ferulic Acid on Deoxynivalenol-Induced Toxicity in IPEC-J2 Cells. Toxins 2022, 14, 275. https://doi.org/10.3390/toxins14040275
Meng X, Yu W, Duan N, Wang Z, Shen Y, Wu S. Protective Effects of Ferulic Acid on Deoxynivalenol-Induced Toxicity in IPEC-J2 Cells. Toxins. 2022; 14(4):275. https://doi.org/10.3390/toxins14040275
Chicago/Turabian StyleMeng, Xiangyi, Wenyan Yu, Nuo Duan, Zhouping Wang, Yingbin Shen, and Shijia Wu. 2022. "Protective Effects of Ferulic Acid on Deoxynivalenol-Induced Toxicity in IPEC-J2 Cells" Toxins 14, no. 4: 275. https://doi.org/10.3390/toxins14040275
APA StyleMeng, X., Yu, W., Duan, N., Wang, Z., Shen, Y., & Wu, S. (2022). Protective Effects of Ferulic Acid on Deoxynivalenol-Induced Toxicity in IPEC-J2 Cells. Toxins, 14(4), 275. https://doi.org/10.3390/toxins14040275