Montane Rattlesnakes in México: Venoms of Crotalus tancitarensis and Related Species within the Crotalus intermedius Group
Abstract
:1. Introduction
2. Results
2.1. SDS-PAGE
2.2. Enzyme Assays
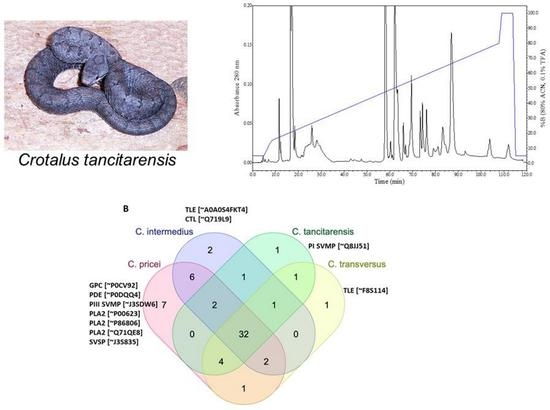
2.3. Reverse-Phase High Performance Liquid Chromatography (RP-HPLC)
2.4. Mass Spectrometry
2.5. Lethal Toxicity (LD50) Assays
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Supplies and Reagents
5.2. Animals and Venoms
5.3. Protein Concentration Determination
5.4. Protein Polyacrylamide Gel Electrophoresis (SDS-PAGE)
5.5. Enzyme Assays
5.6. Reverse-Phase High Performance Liquid Chromatography (RP-HPLC)
5.7. Mass Spectrometry Analysis
5.8. Lethal Toxicity (LD50) Assays
5.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uetz, P.; Freed, P.; Aguilar, R.; Hošek, J. (Eds.) The Reptile Database 2022. Available online: http://www.reptile-database.org (accessed on 30 August 2022).
- Blair, C.; Sánchez-Ramírez, S. Diversity-dependent cladogenesis throughout western México: Evolutionary biogeography of rattlesnakes (Viperidae: Crotalinae: Crotalus and Sistrurus). Mol. Phylogenet. Evol. 2016, 97, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryson, R.W.; Murphy, R.W.; Graham, M.R.; Lathrop, A.; Lazcano, D. Ephemeral Pleistocene woodlands connect the dots for highland rattlesnakes of the Crotalus intermedius group. J. Biogeog. 2011, 38, 2299–2310. [Google Scholar] [CrossRef]
- Colis-Torres, A.; Neri-Castro, E.; Strickland, J.L.; Olvera-Rodríguez, A.; Borja, M.; Calvete, J.; Jones, J.; Parkinson, C.L.; Bañuelos, J.; López de León, J.; et al. Intraspecific venom variation of Mexican West Coast Rattlesnakes (Crotalus basiliscus) and its implications for antivenom production. Biochimie 2022, 192, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Durban, J.; Sanz, L.; Trevisan-Silva, D.; Neri-Castro, E.; Alagón, A.; Calvete, J.J. Integrated venomics and venom gland transcriptome analysis of juvenile and adult Mexican rattlesnakes Crotalus simus, C. tzabcan, and C. culminatus revealed miRNA-modulated ontogenetic shifts. J. Proteome Res. 2017, 16, 3370–3390. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.L.; Smith, C.F.; Mason, A.J.; Borja, M.; Castañeda-Gaytán, G.; Schield, D.R.; Castoe, T.A.; Spencer, C.L.; Smith, L.L.; Trápaga, A.; et al. Evidence for divergent patterns of local selection driving venom variation in Mojave Rattlesnakes (Crotalus scutulatus). Sci. Rep. 2018, 8, 17622. [Google Scholar] [CrossRef] [Green Version]
- Neri-Castro, E.; Zarzosa, V.; Colis-Torres, A.; Fry, B.G.; Olvera-Rodríguez, A.; Jones, J.; Reyes-Velasco, J.; Zamudio, F.; Borja, M.; Alagón, A.; et al. Proteomic and toxicological characterization of the venoms of the most enigmatic group of rattlesnakes: The long-tailed rattlesnakes. Biochimie 2022, 202, 226–236. [Google Scholar] [CrossRef]
- Castoe, T.A.; Parkinson, C.L. Bayesian mixed models and the phylogeny of pitvipers (Viperidae: Serpentes). Mol. Phylogenet. Evol. 2006, 39, 91–110. [Google Scholar] [CrossRef]
- Place, A.J.; Abramson, C.I. A quantitative analysis of the ancestral area of rattlesnakes. J. Herpetol. 2004, 38, 151–156. [Google Scholar] [CrossRef]
- Reyes-Velasco, J.; Meik, J.M.; Smith, E.N.; Castoe, T.A. Phylogenetic relationships of the enigmatic long-tailed rattlesnakes (Crotalus ericsmithi, C. lannomi, and C. stejnegeri). Mol. Phylogenet. Evol. 2013, 69, 524–534. [Google Scholar] [CrossRef]
- Peterson, A.T.; Navarro-Sigüenza, A.G. Alternate species concepts as bases for determining priority conservation areas. Conserv. Biol. 1999, 13, 427–431. [Google Scholar] [CrossRef]
- Alvarado-Díaz, J.; Campbell, J.A. A new montane rattlesnake (Viperidae) from Michoacán, Mexico. Herpetologica 2004, 60, 281–286. [Google Scholar] [CrossRef]
- Campbell, J.A. A confusing specimen of rattlesnake from Cerro Tancítaro, Michoacán, Mexico. Southwest. Nat. 1982, 27, 353. [Google Scholar] [CrossRef]
- Kardong, K.V.; Kiene, T.L.; Bels, V. Evolution of trophic systems in squamates. Neth. J. Zool. 1997, 47, 411–427. [Google Scholar]
- Mackessy, S.P. Venom composition in rattlesnakes: Trends and biological significance. In The Biology of Rattlesnakes; Hayes, W.K., Beaman, K.R., Cardwell, M.D., Bush, S.P., Eds.; Loma Linda University Press: Loma Linda, CA, USA, 2008; pp. 495–510. [Google Scholar]
- Mackessy, S.P. The field of reptile toxinology: Snakes, lizards, and their venoms. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–21. [Google Scholar]
- Smith, C.F.; Mackessy, S.P. Biochemical ecology of venomous snakes. In Handbook of Venoms and Toxins of Reptiles, 2nd ed.; Mackessy, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 147–160. [Google Scholar]
- Zancolli, G.; Calvete, J.J.; Cardwell, M.D.; Greene, H.W.; Hayes, W.K.; Hegarty, M.J.; Herrmann, H.W.; Holycross, A.T.; Lannutti, D.I.; Mulley, J.F.; et al. When one phenotype is not enough: Divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. Proc. Biol. Sci. 2019, 286, 20182735. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.F.; Mackessy, S.P. The effects of hybridization on divergent venom phenotypes: Characterization of venom from Crotalus scutulatus scutulatus × Crotalus oreganus helleri hybrids. Toxicon 2016, 120, 110–123. [Google Scholar] [CrossRef]
- Grabowsky, E.R.; Mackessy, S.P. Predator-prey interactions and venom composition in a high elevation lizard specialist, Crotalus pricei (Twin-spotted Rattlesnake). Toxicon 2019, 170, 28–40. [Google Scholar] [CrossRef]
- Blair, C.; Bryson, R.W., Jr.; Linkem, C.W.; Lazcano, D.; Klicka, J.; McCormack, J.E. Cryptic diversity in the Mexican highlands: Thousands of UCE loci help illuminate phylogenetic relationships, species limits and divergence times of montane rattlesnakes (Viperidae: Crotalus). Mol. Ecol. Res. 2019, 19, 349–365. [Google Scholar] [CrossRef]
- Bryson, R.W.; Murphy, R.W.; Lathrop, A.; Lazcano-Villareal, D. Evolutionary drivers of phylogeographical diversity in the highlands of Mexico: A case study of the Crotalus triseriatus species group of montane rattlesnakes. J. Biogeogr. 2011, 38, 697–710. [Google Scholar] [CrossRef]
- Coblentz, D.; Riitters, K. A quantitative topographic analysis of the Sky Islands: A closer examination of the topography-biodiversity relationship in the Madrean Archipelago. In Connecting Mountain Islands and Desert seas: Biodiversity and Management of the Madrean Archipelago II; Gottfried, G.J., Gebow, B.R., Eskew, L.G., Edminster, C.B., Eds.; USDA Forest Service Proceedings RMRS-P-36, USDA Forest Service: Fort Collins, CO, USA, 2005; pp. 171–175. [Google Scholar]
- Gottfried, G.J.; Hodges, D. Preface. In Connecting Mountain Islands and Desert Seas: Biodiversity and Management of the Madrean Archipelago II; Gottfried, G.J., Gebow, B.R., Eskew, L.G., Edminster, C.B., Eds.; USDA Forest Service Proceedings RMRS-P-36, USDA Forest Service: Fort Collins, CO, USA, 2005; pp. iii–iiv. [Google Scholar]
- Mastratta-Yanes, A.; Moreno-Letelier, A.; Pinero, D.; Jorgensen, T.H.; Emerson, B.C. Biodiversity in the Mexican highlands and the interaction of geology, geography and climate within the Trans-Mexican Volcanic Belt. J. Biogeogr. 2015, 42, 1586–1600. [Google Scholar] [CrossRef]
- Metcalfe, S.E.; O’Hara, S.L.; Caballero, M.; Davies, S.J. Records of late Pleistocene-Holocene climatic change in Mexico—A review. Quat. Sci. Rev. 2000, 19, 699–721. [Google Scholar] [CrossRef]
- Thompson, R.S.; Anderson, K.H. Biomes of western North America at 18,000, 6000 and 0 14C yr BP reconstructed from pollen and packrat midden data. J. Biogeogr. 2000, 27, 555–584. [Google Scholar] [CrossRef]
- Perrigo, A.; Hoorn, C.; Antonelli, A. Why mountains matter for biodiversity. J. Biogeogr. 2019, 47, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Saviola, A.J.; Gandara, A.J.; Bryson, R.W., Jr.; Mackessy, S.P. Venom phenotypes of the Rock Rattlesnake (Crotalus lepidus) and the Ridge-nosed Rattlesnake (Crotalus willardi) from México and the United States. Toxicon 2017, 138, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P. Fractionation of Red Diamond Rattlesnake (Crotalus ruber ruber) venom: Protease, phosphodiesterase, L-amino acid oxidase activities and effects of metal ions and inhibitors on protease activity. Toxicon 1985, 23, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P. Venom ontogeny in the Pacific rattlesnakes Crotalus viridis helleri and Crotalus viridis oreganus. Copeia 1988, 1988, 92–101. [Google Scholar] [CrossRef]
- Mackessy, S.P.; Leroy, J.; Mociño-Deloya, E.; Setser, K.; Bryson, R.W.; Saviola, A.J. Venom ontogeny in the Mexican Lance-headed Rattlesnake (Crotalus polystictus). Toxins 2018, 10, 271. [Google Scholar] [CrossRef] [Green Version]
- Margres, M.J.; Walls, R.; Suntravat, M.; Lucena, S.; Sanchez, E.E.; Rokyta, D.R. Functional characterizations of venom phenotypes in the eastern diamondback rattlesnake (Crotalus adamanteus). Toxicon 2016, 119, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Cohen, K.M.; Harper, D.A.T.; Gibbard, P.L. ICS International Chronostratigraphic Chart 2018/08; International Commission on Stratigraphy, IUGS: Paris, France, 2018. [Google Scholar]
- McDonald, J.A. Phytogeography and history of the alpine–subalpine flora of northeastern Mexico. In Biological Diversity in Mexico: Origins and Distribution; Ramamoorthy, T.P., Bye, R., Lot, A., Fa, J., Eds.; Oxford University Press: New York, NY, USA, 1993; pp. 681–703. [Google Scholar]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Holding, M.L.; Strickland, J.L.; Rautsaw, R.M.; Hofmann, E.P.; Mason, A.J.; Hogan, M.P.; Nystrom, G.S.; Ellsworth, S.A.; Colston, T.J.; Borja, M.; et al. Phylogenetically diverse diets favor more complex venoms in North American pitvipers. Proc. Natl. Acad. Sci. USA 2021, 118, e2015579118. [Google Scholar] [CrossRef]
- Heyborne, W.H.; Mackessy, S.P. Isolation and characterization of a taxon-specific three-finger toxin from the venom of the Green Vinesnake (Oxybelis fulgidus; family Colubridae). Biochimie 2013, 95, 1923–1932. [Google Scholar] [CrossRef]
- Modahl, C.M.; Mrinalini, F.S.E.; Mackessy, S.P. Adaptive evolution of prey-specific three-finger toxins in the Amazon Puffing Snake, Spilotes sulphureus. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181003. [Google Scholar] [CrossRef]
- Pawlak, J.; Mackessy, S.P.; Sixberry, N.M.; Stura, E.A.; Le Du, M.H.; Ménez, R.; Foo, C.S.; Ménez, A.; Nirthanan, S.; Kini, R.M. Irditoxin, a novel covalently linked heterodimeric three-finger toxin with high taxon-specific neurotoxicity. FASEB J. 2009, 23, 534–545. [Google Scholar] [CrossRef] [Green Version]
- Mackessy, S.P.; Saviola, A.J. Understanding biological roles of venoms among the Caenophidia: The importance of rear-fanged snakes. Integr. Comp. Biol. 2016, 56, 1004–1021. [Google Scholar] [CrossRef]
- Saviola, A.J.; Pla, D.; Sanz, L.; Castoe, T.A.; Calvete, J.J.; Mackessy, S.P. Comparative venomics of the Prairie Rattlesnake (Crotalus viridis viridis) from Colorado: Identification of a novel pattern of ontogenetic changes in venom composition and assessment of the immunoreactivity of the commercial antivenom CroFab®. J. Proteom. 2015, 121, 28–43. [Google Scholar] [CrossRef]
- Kishimoto, M.; Takahashi, T. A spectrophotometric microplate assay for l-amino acid oxidase. Anal. Biochem. 2001, 298, 136–139. [Google Scholar] [CrossRef]
- Robinson, K.E.; Holding, M.L.; Whitford, M.D.; Saviola, A.J.; Yates, J.R., III; Clark, R.W. Phenotypic and functional variation in venom and venom resistance of two sympatric rattlesnakes and their prey. J. Evol. Biol. 2021, 34, 1447–1465. [Google Scholar] [CrossRef]
- Kong, A.T.; Leprevost, F.V.; Avtonomov, D.M.; Mellacheruvu, D.; Nesvizhskii, A.I. MSFragger: Ultrafast and comprehensive peptide identification in mass spectrometry–based proteomics. Nat. Methods 2017, 14, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Bartolomé, S.; Medina-Aunon, J.A.; López-García, M.A.; González-Tejedo, C.; Prieto, G.; Navajas, R.; Salazar-Donate, E.; Fernández-Costa, C.; Yates, J.R., III; Albar, J.P. PACOM: A versatile tool for integrating, filtering, visualizing, and comparing multiple large mass spectrometry proteomics data sets. J. Proteome Res. 2018, 17, 1547–1558. [Google Scholar] [CrossRef]
- Serang, O.; Noble, W. A review of statistical methods for protein identification using tandem mass spectrometry. Stat. Interface 2012, 5, 3–20. [Google Scholar]
- The, M.; MacCoss, M.J.; Noble, W.S.; Käll, L. Fast and accurate protein false discovery rates on large-scale proteomics data sets with percolator 3.0. J. Am. Soc. Mass Spectrom. 2016, 27, 1719–1727. [Google Scholar] [CrossRef] [PubMed]








| Species | Thr (nmol/min/mg) | Kal (nmol/min/mg) | MPr (ΔA342 nm/min/mg) |
|---|---|---|---|
| C. tancitarensis n = 4 | 328 ± 87 | 47 ± 21 | 0.34 ± 0.15 |
| C. intermedius n = 2 | 509 ± 630 | 536 ± 697 | 1.38 ± 0.45 |
| C. transversus n = 1 | 681 | 112 | 0.32 |
| C. p. miquihuanus n = 2 | 3236 ± 29 | 5201 ± 196 | 1.19 ± 0.06 |
| C. p. pricei n = 4 | 699 ± 182 | 1055 ± 261 | 1.04 ± 0.04 |
| Species | PLA2 (nmol/min/mg) | LAAO (ΔA492nm/min/mg) | PDE (ΔA400nm/min/mg) |
|---|---|---|---|
| C. tancitarensis n = 2 | 34.6 ± 3.8 | 16.3 ± 2.6 | 0.098 ± 0.052 |
| C. intermedius n = 2 | 53.8 ± 12.4 | -- | -- |
| C. transversus n = 1 | 49.8 | 29.0 | 0.124 |
| C. p. miquihuanus n = 2 | 79.6 ± 11.4 | 16.8 ± 3.3 | 0.232 ± 0.124 |
| C. p. pricei n = 4 | 53.8 ± 10.5 | 21.5 ± 3.6 | 0.037 ± 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowsky, E.R.; Saviola, A.J.; Alvarado-Díaz, J.; Mascareñas, A.Q.; Hansen, K.C.; Yates, J.R., III; Mackessy, S.P. Montane Rattlesnakes in México: Venoms of Crotalus tancitarensis and Related Species within the Crotalus intermedius Group. Toxins 2023, 15, 72. https://doi.org/10.3390/toxins15010072
Grabowsky ER, Saviola AJ, Alvarado-Díaz J, Mascareñas AQ, Hansen KC, Yates JR III, Mackessy SP. Montane Rattlesnakes in México: Venoms of Crotalus tancitarensis and Related Species within the Crotalus intermedius Group. Toxins. 2023; 15(1):72. https://doi.org/10.3390/toxins15010072
Chicago/Turabian StyleGrabowsky, Emily R., Anthony J. Saviola, Javier Alvarado-Díaz, Adrian Quijada Mascareñas, Kirk C. Hansen, John R. Yates, III, and Stephen P. Mackessy. 2023. "Montane Rattlesnakes in México: Venoms of Crotalus tancitarensis and Related Species within the Crotalus intermedius Group" Toxins 15, no. 1: 72. https://doi.org/10.3390/toxins15010072
APA StyleGrabowsky, E. R., Saviola, A. J., Alvarado-Díaz, J., Mascareñas, A. Q., Hansen, K. C., Yates, J. R., III, & Mackessy, S. P. (2023). Montane Rattlesnakes in México: Venoms of Crotalus tancitarensis and Related Species within the Crotalus intermedius Group. Toxins, 15(1), 72. https://doi.org/10.3390/toxins15010072