A 15-Year Retrospective Review of Ciguatera in the Madeira Islands (North-East Atlantic, Portugal)
Abstract
:1. Introduction
2. Ciguatera Poisonings in Madeira Archipelago
3. The Particular Case of Selvagens Islands
4. Occurrence and Distribution of Gambierdiscus
5. Ciguatoxicity and Ciguatoxins Profile in Seafood

| Species | Common Name | Location of Capture | Weight (kg) | Date | Tissue | N2a-CBA | LC-MSMS/HRMS | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Concentration | Toxins | ||||||||
| Seriola rivoliana * | Longfin yellowtail | Selvagens Islands | not specified | 2008 | Flesh | 0.17 µg C-CTX1 eq. kg−1 | Not quantified | C-CTX1 | [16] |
| Seriola fasciata * | Lesser amberjack | Selvagens Islands | not specified | 2008 | Flesh | up to 6.23 µg C-CTX1 eq. kg−1 | Not tested | Not tested | [47] |
| Seriola sp. * | Amberjack | Selvagens Islands | not specified | 2009 | Flesh | 0.08 µg C-CTX1 eq. kg−1 | Not quantified | C-CTX1 | [16] |
| Seriola fasciata ** | Lesser amberjack | Selvagens Islands | 10.0 | March 2009 | Tail muscle | 40.6 µg kg−1 | 35.29 µg kg−1 | CTX-1B at m/z 1111.6, CTX-3C at 1023.5 m/z, CTXs at m/z 1040.6 and 1141.6 | [14] |
| Seriola dumerili ** | Greater amberjack | Selvagens Islands | 70.0 | April 2009 | Several tissues (tail, head, ventral, liver) | 37.3–45.1 µg kg−1 | 33.29–54.35 µg kg−1 | [16] | |
| Seriola fasciata * | Lesser amberjack | Selvagens Islands | 37.0 | November 2008 | Flesh | 1.4 µg C-CTX1 eq. kg−1 | 0.84 µg C-CTX1 eq. kg−1 | C-CTX1 and three C-CTX congeners of m/z 1157, m/z 1127 and m/z 1123 | [45] |
| Gymnothorax unicolor | Brown moray | Selvagens Islands | 1.2 | December 2013 | Flesh | 0.039 µg CTX1B eq. kg−1 | CTX at m/z 1127.6, dihydro-CTX2 analogue at m/z 1115.6 | [57] | |
| Muraena augusti | Morey eel | Desertas Islands | 1.7 | November 2013 | Flesh | 0.065 µg CTX1B eq. kg−1 | [57] | ||
| Muraena helena | Mediterranean moray | Desertas Islands | 1.3 | November 2013 | Flesh | 0.083 ± 0.014 µg CTX1B eq. kg−1 | [57] | ||
| Pagrus pagrus * | Red Porgy | Selvagens Islands | 4.0 | December 2016 | Flesh | Not tested | 0.76 µg C-CTX1 eq. kg−1 | C-CTX1 and a hydroxyl metabolite at m/z 1181.7 | [48] |
| Epinephelus marginatus | Dusky grouper | Selvagens Islands | 19.5 | December 2016 | Flesh | Not tested | 0.05 µg C-CTX1 eq. kg−1 | C-CTX1 | [17] |
| Mycteroperca fusca | Island grouper | Selvagens Islands | 4.5 | December 2016 | Flesh | Not tested | 0.25 µg C-CTX1 eq. kg−1 | [17] | |
| Bodianus scrofa | Barred hogfish | Selvagens Islands | 1.7–0.8 | November 2017 | Flesh | Not tested | 0.11–0.06 µg C-CTX1 eq. kg−1 | [17] | |
| Balistes capriscus | Grey triggerfish | Selvagens Islands | 2.0 | November 2017 | Flesh | Not tested | 0.03 µg C-CTX1 eq. kg−1 | [17] | |
| Sparisoma cretense | Parrotfish | Selvagens Islands | 0.9–0.4 | September 2018 | Flesh | 0.006–0.04 µg CTX1B eq. kg−1 | <LOQ | [46] | |
| Diplodus cervinus | Zebra seabream | Selvagens Islands | 2.8 | September 2018 | Flesh | 0.37 µg CTX1B eq. kg−1 | <LOQ | C-CTXs congeners, C-CTX-1157 | [46] |
| Bodianus scrofa | Barred hogfish | Selvagens Islands | 3.0–1.1 | September 2018 | Flesh | 0.04–0.75 µg CTX1B eq. kg−1 | 0.08–0.48 µg C-CTX1 eq. kg−1 | C-CTX1 | [46] |
| Balistes capriscus | Grey triggerfish | Selvagens Islands | 2.6–0.6 | September 2018 | Flesh | <LOQ–0.06 µg CTX1B eq. kg−1 | <LOQ–0.09 µg C-CTX1 eq. kg−1 | C-CTX1 | [46] |
| Serranus atricauda | Blacktail comber | Selvagens Islands | 0.3–0.2 | September 2018 | Flesh | 0.006–0.02 µg CTX1B eq. kg−1 | <LOQ | [46] | |
| Sphyraena viridensis | Yellowmouth barracuda | Selvagens Islands | 6.0–2.2 | September 2018 | Flesh | up to 0.22 µg CTX1B eq. kg−1 | up to 0.14 µg C-CTX1 eq. kg−1 | C-CTX1 | [46] |
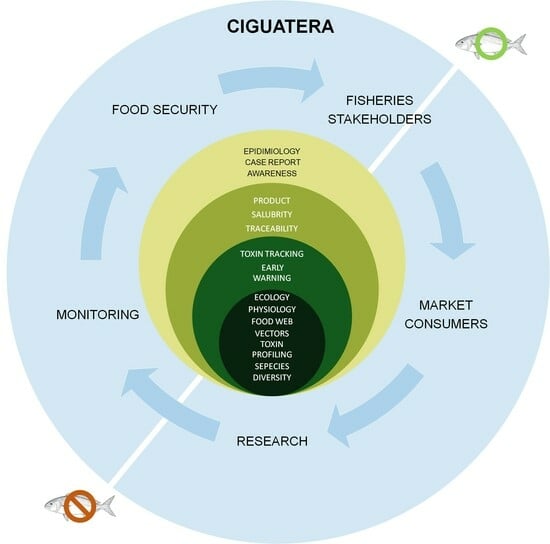
6. Ciguatera Risk Management
7. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, R.J.; Holmes, M.J. Origin and Transfer of Toxins Involved in Ciguatera. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1993, 106, 615–628. [Google Scholar] [CrossRef]
- Mudge, E.M.; Miles, C.O.; Ivanova, L.; Uhlig, S.; James, K.S.; Erdner, D.L.; Fæste, C.K.; McCarron, P.; Robertson, A. Algal Ciguatoxin Identified as Source of Ciguatera Poisoning in the Caribbean. Chemosphere 2023, 330, 138659. [Google Scholar] [CrossRef] [PubMed]
- Kibler, S.R.; Tester, P.A.; Kunkel, K.E.; Moore, S.K.; Litaker, R.W. Effects of ocean warming on growth and distribution of dinoflagellates associated with ciguatera fish poisoning in the Caribbean. Ecol. Model. 2015, 316, 194–210. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.I.; Darius, H.T.; Quod, J.-P.; Tester, P.A. Ciguatera Poisonings: A Global Review of Occurrences and Trends. Harmful Algae 2021, 102, 101873. [Google Scholar] [CrossRef] [PubMed]
- Aligizaki, K.; Nikolaidis, G. Morphological identification of two tropical dinoflagellates of the genera Gambierdiscus and Sinophysis in the Mediterranean Sea. J. Biol. Res.-Thessalon. 2008, 9, 75–82. [Google Scholar]
- Fraga, S.; Rodríguez, F.; Caillaud, A.; Diogène, J.; Raho, N.; Zapata, M. Gambierdiscus excentricus sp. nov. (Dinophyceae), a benthic toxic dinoflagellate from the Canary Islands (NE Atlantic Ocean). Harmful Algae 2011, 11, 10–22. [Google Scholar] [CrossRef]
- Reverté, L.; Toldrà, A.; Andree, K.B.; Fraga, S.; de Falco, G.; Campàs, M.; Diogène, J. Assessment of cytotoxicity in ten strains of Gambierdiscus australes from Macaronesian Islands by neuro-2a cell-based assays. J. Appl. Phycol. 2018, 30, 2447–2461. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Kretzschmar, A.L.; Kaufmann, M.J.; Murray, S.A. Morphological and molecular phylogenetic identification and record verification of Gambierdiscus excentricus (Dinophyceae) from Madeira Island (NE Atlantic Ocean). Mar. Biodivers. Rec. 2019, 12, 16. [Google Scholar] [CrossRef]
- Godinho, L.; Soliño, L.; Churro, C.; Timoteo, V.; Santos, C.; Gouveia, N.; Diogène, J.; Reis Costa, P. Distribution, Identification and Cytotoxicity of Gambierdiscus (Dinophyceae) in the Atlantic Selvagens Islands (Madeira, Portugal): A Ciguatera Gateway to Europe. Eur. J. Phycol. 2023, 58, 156–168. [Google Scholar] [CrossRef]
- Ikehara, T.; Kuniyoshi, K.; Oshiro, N.; Yasumoto, T. Biooxidation of Ciguatoxins Leads to Species-Specific Toxin Profiles. Toxins 2017, 9, 205. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. Off. J. Eur. Union 2004, L139, 55–205. [Google Scholar]
- European Commission. Commission Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. Off. J. Eur. Union 2004, L226, 83–127. [Google Scholar]
- Perez-Arellano, J.L.; Luzardo, O.P.; Brito, A.P.; Cabrera, M.H.; Zumbado, M.; Carranza, C.; Angel-Moreno, A.; Dickey, R.W.; Boada, L.D. Ciguatera fish poisoning, Canary Islands. Emerg. Infect. Dis. 2005, 11, 1981–1982. [Google Scholar] [CrossRef]
- Otero, P.; Pérez, S.; Alfonso, A.; Vale, C.; Rodríguez, P.; Gouveia, N.N.; Gouveia, N.; Delgado, J.; Vale, P.; Hirama, M.; et al. First toxin profile of ciguateric fish in Madeira Arquipelago (Europe). Anal. Chem. 2010, 82, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, N.N.; Vale, P.; Gouveia, N.; Delgado, J. Primeiro registo da ocorrência de episódios do tipo ciguatérico no arquipélago da Madeira. In Algas Tóxicas e Biotoxinas nas Águas da Península Ibérica-2009; Costa, P.R., Botelho, M.J., Rodrigues, S.M., Palma, A.S., Moita, M.T., Eds.; IPIMAR: Lisboa, Potugal, 2009; pp. 152–157. [Google Scholar]
- Boada, L.D.; Zumbado, M.; Luzardo, O.R.; Almeida-Gonzalez, M.; Plakas, S.M.; Granade, H.R.; Abraham, A.; Jester, E.L.E.; Dickey, R.W. Ciguatera fish poisoning on the West Africa Coast: An emerging risk in the Canary Islands (Spain). Toxicon 2010, 56, 1516–1519. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Estevez, P.; Castro, D.; Soliño, L.; Gouveia, N.; Santos, C.; Rodrigues, S.; Leao, J.; Gago-Martínez, A. New Insights into the Occurrence and Toxin Profile of Ciguatoxins in Selvagens Islands (Madeira, Portugal). Toxins 2018, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Soliño, L.; Costa, P.R. Global Impact of Ciguatoxins and Ciguatera Fish Poisoning on Fish, Fisheries and Consumers. Environ. Res. 2020, 182, 109111. [Google Scholar] [CrossRef]
- Barton, E.D.; Arístegui, J.; Tett, P.; Cantón, M.; García-Braun, J.; Hernández-León, S.; Nykjaer, L.; Almeida, C.; Almunia, J.; Ballesteros, S.; et al. The transition zone of the Canary Current upwelling region. Prog. Oceanogr. 1998, 41, 455–504. [Google Scholar] [CrossRef]
- DLR n.º 8/2022/M. Assembleia Legislativa da Região Autónoma da Madeira, Aprova o Novo Regime Jurídico da Reserva Natural das Ilhas Selvagens. Diário da República n.º 85/2022, Série I de 2022-05-03. pp. 5–14. Available online: https://files.dre.pt/1s/2022/05/08500/0000500014.pdf (accessed on 28 September 2023). (In Portuguese).
- Friedlander, A.; Ballesteros, E.; Clemente, S.; Estep, A.; Rose, P.; Shepard, M. Marine Biodiversity and Ecosystem Health of Ilhas Selvagens, Portugal; Scientific Report to the Government of Portugal and the Regional Government of Madeira; National Geographic Pristine Seas: Washington, DC, USA, 2016; pp. 1–64. [Google Scholar]
- Almada, F.; Abecasis, D.; Villegas-Ríos, D.; Henriques, S.; Pais, M.P.; Batista, M.; Horta e Costa, B.; Martins, J.; Tojeira, I.; Rodrigues, N.V.; et al. Ichthyofauna of the Selvagens Islands. Do small coastal areas show high species richness in the northeastern Atlantic? Mar. Biol. Res. 2015, 11, 49–61. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Ballesteros, E.; Clemente, S.; Gonçalves, E.J.; Estep, A.; Rose, P.; Sala, E. Contrasts in the Marine Ecosystem of Two Macaronesian Islands: A Comparison between the Remote Selvagens Reserve and Madeira Island. PLoS ONE 2017, 12, e0187935. [Google Scholar] [CrossRef]
- Yasumoto, T.; Nakajima, I.; Bagnis, R.; Adachi, R. Finding of a Dinoflagellate as a Likely Culprit of Ciguatera. Bull. Jpn. Soc. Sci. Fis. 1977, 43, 1021–1026. [Google Scholar] [CrossRef]
- Adachi, R.; Fukuyo, Y. The Thecal Structure of a Marine Toxic Dinoflagellate Gambierdiscus toxicus gen. et sp. nov. Collected in a Ciguatera-endemic Area. Bull. Jpn. Soc. Sci. Fis. 1979, 45, 67–71. [Google Scholar] [CrossRef]
- Rodríguez, F.; Fraga, S.; Ramilo, I.; Rial, P.; Figueroa, R.I.; Riobó, P.; Bravo, I. Canary Islands (NE Atlantic) as a biodiversity ‘hotspot’ of Gambierdiscus: Implications for future trends of ciguatera in the area. Harmful Algae 2017, 67, 131–143. [Google Scholar] [CrossRef]
- Larsson, M.E.; Laczka, O.F.; Harwood, D.T.; Lewis, R.J.; Himaya, S.W.A.; Murray, S.A.; Doblin, M.A. Toxicology of Gambierdiscus spp. (Dinophyceae) from Tropical and Temperate Australian Waters. Mar. Drugs 2018, 16, 7. [Google Scholar] [CrossRef]
- Tudó, À.; Gaiani, G.; Varela, M.R.; Tsumuraya, T.; Andree, K.B.; Fernández-Tejedor, M.; Campàs, M.; Diogène, J. Further advance of Gambierdiscus species in the Canary Islands, with the first report of Gambierdiscus belizeanus. Toxins 2020, 12, 692. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M.; AlgaeBase. World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2023; Available online: https://www.algaebase.org (accessed on 28 September 2023).
- Fraga, S.; Rodríguez, F. Genus Gambierdiscus in the Canary Islands (NE Atlantic Ocean) with description of Gambierdiscus silvae sp. nov., a new potentially toxic epiphytic benthic dinoflagellate. Protist 2014, 165, 839–853. [Google Scholar] [CrossRef]
- Silva, E.S. Contribution a L’étude du Microplancton de Dakar et des Régions Maritimes Voisines. Bull. Inst. Fr. Afr. Noire. Ser. A Sci. Nat. 1956, 18, 335–371. [Google Scholar]
- Tester, P.A.; Litaker, R.W.; Berdalet, E. Climate Change and Harmful Benthic Microalgae. Harmful Algae 2020, 91, 101655. [Google Scholar] [CrossRef] [PubMed]
- Aligizaki, K.; Nikolaidis, G.; Fraga, S. Is Gambierdiscus expanding to new areas? Harmful Algae News 2008, 36, 6–7. [Google Scholar]
- Rossignoli, A.E.; Tudó, A.; Bravo, I.; Díaz, P.A.; Diogène, J.; Riobó, P. Toxicity Characterisation of Gambierdiscus Species from the Canary Islands. Toxins 2020, 12, 134. [Google Scholar] [CrossRef]
- Estevez, P.; Sibat, M.; Leão-Martins, J.M.; Tudó, A.; Rambla-Alegre, M.; Aligizaki, K.; Diogène, J.; Gago-Martinez, A.; Hess, P. Use of Mass Spectrometry to Determine the Diversity of Toxins Produced by Gambierdiscus and Fukuyoa Species from Balearic Islands and Crete (Mediterranean Sea) and the Canary Islands (Northeast Atlantic). Toxins 2020, 12, 305. [Google Scholar] [CrossRef] [PubMed]
- Gaiani, G.; Cucchi, F.; Toldrà, A.; Andree, K.B.; Rey, M.; Tsumuraya, T.; O’Sullivan, C.K.; Diogène, J.; Campàs, M. Electrochemical Biosensor for the Dual Detection of Gambierdiscus australes and Gambierdiscus excentricus in Field Samples. First Report of G. excentricus in the Balearic Islands. Sci. Total Environ. 2022, 806, 150915. [Google Scholar] [CrossRef] [PubMed]
- Aligizaki, K.; Battocchi, C.; Penna, A.; Rodríguez Hernández, F.; Arsenakis, M.; Fraga, S. Diversity of potentially toxic benthic dinoflagellates in southern Europe. In Proceedings of the 14th International Conference on Harmful Algae, Crete, Greece, 1 November 2010. [Google Scholar]
- Bravo, I.; Rodriguez, F.; Ramilo, I.; Rial, P.; Fraga, S. Ciguatera-Causing Dinoflagellate Gambierdiscus spp. (Dinophyceae) in a Subtropical Region of North Atlantic Ocean (Canary Islands): Morphological Characterization and Biogeography. Toxins 2019, 11, 423. [Google Scholar] [CrossRef]
- Tester, P.A.; Kibler, S.R.; Holland, W.C.; Usup, G.; Vandersea, M.W.; Leaw, C.P.; Teen, L.P.; Larsen, J.; Mohammad-Noor, N.; Faust, M.A.; et al. Sampling Harmful Benthic Dinoflagellates: Comparison of Artificial and Natural Substrate Methods. Harmful Algae 2014, 39, 8–25. [Google Scholar] [CrossRef]
- Fernández-Zabala, J.; Tuya, F.; Amorim, A.; Soler-Onís, E. Benthic Dinoflagellates: Testing the Reliability of the Artificial Substrate Method in the Macaronesian Region. Harmful Algae 2019, 87, 101634. [Google Scholar] [CrossRef]
- Estevez, P.; Castro, D.; Leão-Martins, J.M.; Sibat, M.; Tudó, A.; Dickey, R.; Diogene, J.; Hess, P.; Gago-Martinez, A. Toxicity Screening of a Gambierdiscus australes Strain from the Western Mediterranean Sea and Identification of a Novel Maitotoxin Analogue. Mar. Drugs 2021, 19, 460. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, M.J.; Santos, F.; Maranhão, M. Checklist of Nanno- and Microphytoplankton off Madeira Island (Northeast Atlantic) with Some Historical Notes. Nov. Hedwigia 2015, 101, 205–232. [Google Scholar] [CrossRef]
- Pisapia, F.; Holland, W.C.; Hardison, D.R.; Litaker, R.W.; Fraga, S.; Nishimura, T.; Adachi, M.; Nguyen-Ngoc, L.; Séchet, V.; Amzil, Z.; et al. Toxicity Screening of 13 Gambierdiscus Strains Using Neuro-2a and Erythrocyte Lysis Bioassays. Harmful Algae 2017, 63, 173–183. [Google Scholar] [CrossRef]
- Pisapia, F.; Sibat, M.; Herrenknecht, C.; Lhaute, K.; Gaiani, G.; Ferron, P.-J.; Fessard, V.; Fraga, S.; Nascimento, S.M.; Litaker, R.W.; et al. Maitotoxin-4, a Novel MTX Analog Produced by Gambierdiscus excentricus. Mar. Drugs 2017, 15, 220. [Google Scholar] [CrossRef]
- Estevez, P.; Castro, D.; Manuel Leao, J.; Yasumoto, T.; Dickey, R.; Gago-Martinez, A. Implementation of Liquid Chromatography Tandem Mass Spectrometry for the Analysis of Ciguatera Fish Poisoning in Contaminated Fish Samples from Atlantic Coasts. Food Chem. 2019, 280, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.R.; Estevez, P.; Soliño, L.; Castro, D.; Rodrigues, S.M.; Timoteo, V.; Leao-Martins, J.M.; Santos, C.; Gouveia, N.; Diogène, J.; et al. An Update on Ciguatoxins and CTX-like Toxicity in Fish from Different Trophic Levels of the Selvagens Islands (NE Atlantic, Madeira, Portugal). Toxins 2021, 13, 580. [Google Scholar] [CrossRef]
- Caillaud, A.; Eixarch, H.; de la Iglesia, P.; Rodriguez, M.; Dominguez, L.; Andree, K.B.; Diogène, J. Towards the Standardisation of the Neuroblastoma (Neuro-2a) Cell-Based Assay for Ciguatoxin-like Toxicity Detection in Fish: Application to Fish Caught in the Canary Islands. Food Addit. Contam. Part A 2012, 29, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Estevez, P.; Castro, D.; Pequeño-Valtierra, A.; Leao, J.M.; Vilariño, O.; Diogène, J.; Gago-Martínez, A. An Attempt to Characterize the Ciguatoxin Profile in Seriola Fasciata Causing Ciguatera Fish Poisoning in Macaronesia. Toxins 2019, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.E.; De Angelis, N. (Eds.) Volume 4: Bony Fishes Part 2 (Perciformes to Tetradontiformes) and Sea Turtles. In The Living Marine Resources of the Eastern Central Atlantic; FAO Species Identification Guide for Fishery Purposes; FAO: Rome, Italy, 2016; pp. 2343–3124. [Google Scholar]
- Silva, M.; Rodriguez, I.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. First Report of Ciguatoxins in Two Starfish Species: Ophidiaster ophidianus and Marthasterias glacialis. Toxins 2015, 7, 3740–3757. [Google Scholar] [CrossRef] [PubMed]
- Estevez, P.; Sibat, M.; Leão-Martins, J.M.; Reis Costa, P.; Gago-Martínez, A.; Hess, P. Liquid Chromatography Coupled to High-Resolution Mass Spectrometry for the Confirmation of Caribbean Ciguatoxin-1 as the Main Toxin Responsible for Ciguatera Poisoning Caused by Fish from European Atlantic Coasts. Toxins 2020, 12, 267. [Google Scholar] [CrossRef]
- Estevez, P.; Oses Prieto, J.; Burlingame, A.; Gago Martinez, A. Characterization of the Ciguatoxin Profile in Fish Samples from the Eastern Atlantic Ocean Using Capillary Liquid Chromatography-High Resolution Mass Spectrometry. Food Chem. 2023, 418, 135960. [Google Scholar] [CrossRef]
- Lewis, R.J.; Sellin, M.; Poli, M.A.; Norton, R.S.; MacLeod, J.K.; Sheil, M.M. Purification and Characterization of Ciguatoxins from Moray Eel (Lycodontis javanicus, Muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar] [CrossRef]
- FDA. Fish and Fishery Products Hazards and Controls Guidance. 2022. Available online: https://www.fda.gov/food/seafood-guidance-documents-regulatory-information/fish-and-fishery-products-hazards-and-controls (accessed on 28 September 2023).
- Raposo-García, S.; Castro, D.; Lence, E.; Estévez, P.; Leão, J.M.; González-Bello, C.; Gago-Martínez, A.; Louzao, M.C.; Vale, C.; Botana, L.M. In Silico Simulations and Functional Cell Studies Evidence Similar Potency and Distinct Binding of Pacific and Caribbean Ciguatoxins. Expo. Health 2022, 15, 641–660. [Google Scholar] [CrossRef]
- Yokozeki, T.; Hama, Y.; Fujita, K.; Igarashi, T.; Hirama, M.; Tsumuraya, T. Evaluation of Relative Potency of Calibrated Ciguatoxin Congeners by Near-Infrared Fluorescent Receptor Binding and Neuroblastoma Cell-Based Assays. Toxicon 2023, 230, 107161. [Google Scholar] [CrossRef]
- Tudó, À.; Rambla-Alegre, M.; Flores, C.; Sagristà, N.; Aguayo, P.; Reverté, L.; Campàs, M.; Gouveia, N.; Santos, C.; Andree, K.B.; et al. Identification of New CTX Analogues in Fish from the Madeira and Selvagens Archipelagos by Neuro-2a CBA and LC-HRMS. Mar. Drugs 2022, 20, 236. [Google Scholar] [CrossRef]
- Lewis, R.J. The changing face of ciguatera. Toxicon 2001, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Henao, J.A.; García-Álvarez, N.; Fernández, A.; Saavedra, P.; Silva Sergent, F.; Padilla, D.; Acosta-Hernández, B.; Martel Suárez, M.; Diogène, J.; Real, F. Predictive Score and Probability of CTX-like Toxicity in Fish Samples from the Official Control of Ciguatera in the Canary Islands. Sci. Total Environ. 2019, 673, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Gobierno de Canarias. Guía del Protocolo de Control del Cambio Climático, de las Especies Exóticas y Exóticas Invasoras, y de la Ciguatoxina en los Productos de la Pesca Extractiva en los Puntos de Primera Venta Autorizados en Canarias. 2023. Available online: https://www.gobiernodecanarias.org/cmsgobcan/export/sites/pesca/galerias/doc/control-calidad-productos/Guia-Protocolo-Ciguat.-y-Exoticas-Rev.3.pdf (accessed on 28 September 2023).
- Castle, J.W.; Rodgers, J.H., Jr. Hypothesis for the role of toxin-producing algae in Phanerozoic mass extinctions based on evidence from the geologic record and modern environments. Environ. Geosci. 2009, 16, 1–23. [Google Scholar] [CrossRef]
- Pyenson, N.D.; Gutstein, C.S.; Parham, J.F.; Le Roux, J.P.; Chavarría, C.C.; Little, H.; Metallo, A.; Rossi, V.; Valenzuela-Toro, A.M.; Velez-Juarbe, J.; et al. Repeated mass strandings of Miocene marine mammals from Atacama Region of Chile point to sudden death at sea. Proc. Royal Soc. B 2014, 281, 20133316. [Google Scholar] [CrossRef] [PubMed]
- Mullins, M.E. Ciguatera Fish Poisoning in the Age of Discovery and the Age of Enlightenment. Clin. Toxicol. 2022, 60, 392–396. [Google Scholar] [CrossRef]
- Cruz, R.C.; Costa, P.R.; Vinga, S.; Krippahl, L.; Lopes, M.B. A Review of Recent Machine Learning Advances for Forecasting Harmful Algal Blooms and Shellfish Contamination. J. Mar. Sci. Eng. 2021, 9, 283. [Google Scholar] [CrossRef]
- Cruz, R.C.; Costa, P.R.; Krippahl, L.; Lopes, M.B. Forecasting Biotoxin Contamination in Mussels across Production Areas of the Portuguese Coast with Artificial Neural Networks. Knowl.-Based Syst. 2022, 257, 109895. [Google Scholar] [CrossRef]
- Parsons, M.L.; Brandt, A.L.; Ellsworth, A.; Leynse, A.K.; Rains, L.K.; Anderson, D.M. Assessing the Use of Artificial Substrates to Monitor Gambierdiscus Populations in the Florida Keys. Harmful Algae 2017, 68, 52–66. [Google Scholar] [CrossRef]
- MGnify. Amplicon Sequencing of Tara Oceans DNA Samples Corresponding to Size Fractions for Protists. Sampling Event Dataset. 2022. Available online: https://www.gbif.org/dataset/d596fccb-2319-42eb-b13b-986c932780ad (accessed on 4 October 2023).
- Pottier, I.; Lewis, R.J.; Vernoux, J.-P. Ciguatera Fish Poisoning in the Caribbean Sea and Atlantic Ocean: Reconciling the Multiplicity of Ciguatoxins and Analytical Chemistry Approach for Public Health Safety. Toxins 2023, 15, 453. [Google Scholar] [CrossRef]
- Gaiani, G.; Diogène, J.; Campàs, M. Addressing Ciguatera Risk Using Biosensors for the Detection of Gambierdiscus and Ciguatoxins. In Biosensors for the Marine Environment; Regan, F., Hansen, P.D., Barceló, D., Eds.; The Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2023; Volume 122. [Google Scholar] [CrossRef]
- Castro, D.; Estevez, P.; Leao-Martins, J.M.; Dickey, R.W.; García-Álvarez, N.; Real, F.; Costa, P.R.; Gago-Martínez, A. Preparation of Ciguatoxin Reference Materials from Canary Islands (Spain) and Madeira Archipelago (Portugal) Fish. J. Mar. Sci. Eng. 2022, 10, 835. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, P.R.; Churro, C.; Rodrigues, S.M.; Frazão, B.; Barbosa, M.; Godinho, L.; Soliño, L.; Timóteo, V.; Gouveia, N. A 15-Year Retrospective Review of Ciguatera in the Madeira Islands (North-East Atlantic, Portugal). Toxins 2023, 15, 630. https://doi.org/10.3390/toxins15110630
Costa PR, Churro C, Rodrigues SM, Frazão B, Barbosa M, Godinho L, Soliño L, Timóteo V, Gouveia N. A 15-Year Retrospective Review of Ciguatera in the Madeira Islands (North-East Atlantic, Portugal). Toxins. 2023; 15(11):630. https://doi.org/10.3390/toxins15110630
Chicago/Turabian StyleCosta, Pedro Reis, Catarina Churro, Susana Margarida Rodrigues, Bárbara Frazão, Miguel Barbosa, Lia Godinho, Lucía Soliño, Viriato Timóteo, and Neide Gouveia. 2023. "A 15-Year Retrospective Review of Ciguatera in the Madeira Islands (North-East Atlantic, Portugal)" Toxins 15, no. 11: 630. https://doi.org/10.3390/toxins15110630
APA StyleCosta, P. R., Churro, C., Rodrigues, S. M., Frazão, B., Barbosa, M., Godinho, L., Soliño, L., Timóteo, V., & Gouveia, N. (2023). A 15-Year Retrospective Review of Ciguatera in the Madeira Islands (North-East Atlantic, Portugal). Toxins, 15(11), 630. https://doi.org/10.3390/toxins15110630