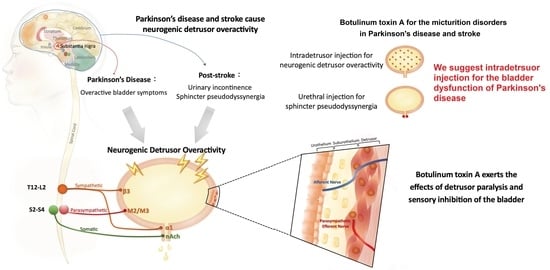
Role of Urological Botulinum Toxin-A Injection for Overactive Bladder and Voiding Dysfunction in Patients with Parkinson’s Disease or Post-Stroke
Abstract
:1. Introduction
2. Brain–Bladder Circuit
3. Bladder Dysfunction in PD Patients
4. UI in Post-Stroke Patients
5. Application of BoNT-A in LUTD
5.1. Structure and Function of BoNT-A
5.2. Biological Effects
6. Urological Injection Techniques of BoNT-A
6.1. Dosage
6.2. The Technique in Bladder Injection
6.3. The Technique in Urethral Sphincter Injection
7. Clinical Efficacy of BoNT-A Treatment in PD
8. Clinical Role of BoNT-A Treatment in Post-Stroke
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuo, H.C. Clinical application of botulinum neurotoxin in lower-urinary-tract disease and dysfunctions: Where are we now and what more can we do? Toxins 2022, 14, 498. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Chen, S.F.; Kuo, H.C. Frontiers in the clinical applications of botulinum toxin A as treatment for neurogenic lower urinary tract dysfunction. Int. Neurourol. J. 2020, 24, 301–312. [Google Scholar] [CrossRef]
- Chiang, C.H.; Chen, S.F.; Kuo, H.C. Video-urodynamic characteristics of lower urinary tract dysfunction in patients with chronic brain disorders. Neurourol. Urodyn. 2022, 41, 255–263. [Google Scholar] [CrossRef]
- Ogawa, T.; Sakakibara, R.; Kuno, S.; Ishizuka, O.; Kitta, T.; Yoshimura, N. Prevalence and treatment of LUTS in patients with Parkinson disease or multiple system atrophy. Nat. Rev. Urol. 2017, 14, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Jiang, Y.H.; Harnod, T.; Lee, H.T.; Kuo, H.C. Stroke and lower urinary tract symptoms: A neurosurgical view. Urol. Sci. 2019, 30, 8–13. [Google Scholar]
- Zillioux, J.; Slopnick, E.A.; Vasavada, S.P. Third-line therapy for overactive bladder in the elderly: Nuances and considerations. Neurourol. Urodyn. 2022, 41, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.J.; Gomelsky, A.; Souter, L.; Vasavada, S.P. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline Amendment 2019. J. Urol. 2019, 202, 558–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillespie, J.I.; van Koeveringe, G.A.; de Wachter, S.G.; de Vente, J. On the origins of the sensory output from the bladder: The concept of afferent noise. BJU Int. 2009, 103, 1324–1333. [Google Scholar] [CrossRef]
- Sakakibara, R.; Tateno, F.; Nagao, T.; Yamamoto, T.; Uchiyama, T.; Yamanishi, T.; Yano, M.; Kishi, M.; Tsuyusaki, Y.; Aiba, Y. Bladder function of patients with Parkinson’s disease. Int. J. Urol. 2014, 21, 638–646. [Google Scholar] [CrossRef]
- Kitta, T.; Kakizaki, H.; Furuno, T.; Moriya, K.; Tanaka, H.; Shiga, T.; Tamaki, N.; Yabe, I.; Sasaki, H.; Nonomura, K. Brain activation during detrusor overactivity in patients with Parkinson’s disease: A PET study. J. Urol. 2006, 175, 994–998. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lee, W.C.; Chuang, Y.C.; Tsai, C.N.; Yu, C.C.; Wang, H.J.; Su, C.H. Using a rat model to translate and explore the pathogenesis of ketamine-induced cystitis. Urol. Sci. 2022, 33, 176–181. [Google Scholar]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural. Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, G.; Liu, J. Autonomic dysfunction in Parkinson’s disease: Implication for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 2020, 134, 104700. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, R.; Panicker, J.; Finazzi-Agro, E.; Iacovelli, V.; Bruschini, H.; Parkinson’s Disease Subcomittee. A guideline for the management of bladder dysfunction in Parkinson’s disease and other gait disorders. Neurourol. Urodyn. 2016, 35, 551–563. [Google Scholar] [CrossRef]
- McDonald, C.; Winge, K.; Burn, D.J. Lower urinary tract symptoms in Parkinson’s disease: Prevalence, aetiology and management. Park. Relat. Disord. 2017, 35, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Li, F.F.; Cui, Y.S.; Yan, R.; Cao, S.S.; Feng, T. Prevalence of lower urinary tract symptoms, urinary incontinence and retention in Parkinson’s disease: A systematic review and meta-analysis. Front. Aging Neurosci. 2022, 14, 977572. [Google Scholar] [CrossRef]
- Wang, J.; Cao, R.; Huang, T.; Liu, C.; Fan, Y. Urinary dysfunction is associated with nigrostriatal dopaminergic degeneration in early and untreated patients with Parkinson’s disease. Park. Dis. 2020, 2020, 4981647. [Google Scholar] [CrossRef]
- Mito, Y.; Yabe, I.; Yaguchi, H.; Takei, T.; Terae, S.; Tajima, Y. Relation of overactive bladder with motor symptoms and dopamine transporter imaging in drug-naïve Parkinson’s disease. Park. Relat. Disord. 2018, 50, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Tkaczynska, Z.; Pilotto, A.; Becker, S.; Gräber-Sultan, S.; Berg, D.; Liepelt-Scarfone, I. Association between cognitive impairment and urinary dysfunction in Parkinson’s disease. J. Neural. Transm. 2017, 124, 543–550. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, K.W.; Heo, K.O.; Chung, S.J.; Choo, M.S. Urodynamic study for distinguishing multiple system atrophy from Parkinson disease. Neurology 2019, 93, e946–e953. [Google Scholar] [CrossRef]
- Vurture, G.; Peyronnet, B.; Palma, J.A.; Sussman, R.D.; Malacarne, D.R.; Feigin, A.; Palmerola, R.; Rosenblum, N.; Frucht, S.; Kaufmann, H.; et al. Urodynamic mechanisms underlying overactive bladder symptoms in patients with Parkinson disease. Int. Neurourol. J. 2019, 23, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, T.; Ma, J.; Ou, T. Evaluation of neurogenic bladder outlet obstruction mimicking sphincter bradykinesia in male patients with Parkinson’s disease. BMC Neurol. 2021, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Wafa, H.A.; Wolfe, C.D.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of stroke in Europe: Thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life year. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, B.O.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. Prevalence, incidence, and mortality of stroke in China: Results from a nationwide population-based survey of 480687 adults. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef]
- Holroyd, S. Urinary incontinence after stroke. Br. J. Community Nurs. 2019, 24, 590–594. [Google Scholar] [CrossRef]
- Akkoç, Y.; Yıldız, N.; Bardak, A.N.; Ersöz, M.; Tunç, H.; Köklü, K.; Alemdaroğlu, E.; Güler, A.; Şaşmaz, E.; Doğan, A.; et al. The course of post-stroke bladder problems and their relation with functional and mental status and quality of life: A six-month, prospective, multicenter study. Turk. J. Phys. Med. Rehabil. 2019, 65, 335–342. [Google Scholar] [CrossRef]
- Chohan, S.A.; Venkatesh, P.K.; How, C.H. Long-term complications of stroke and secondary prevention: An overview for primary care physicians. Singap. Med. J. 2019, 60, 616–620. [Google Scholar] [CrossRef] [Green Version]
- Turhan, N.; Atalay, A.; Atabek, K. Impact of stroke etiology, lesion location and aging on post-stroke urinary incontinence as a predictor of functional recovery. Int. J. Rehabil. Res. 2006, 29, 335–338. [Google Scholar] [CrossRef]
- Cai, W.; Wang, J.; Wang, L.; Wang, J.; Guo, L. Prevalence and risk factors of urinary incontinence for the post-stroke inpatients in Southern China. Neurol. Urodyn. 2015, 34, 231–235. [Google Scholar] [CrossRef]
- Bizovičar, N.; Mali, B.; Goljar, N. Clinical risk factors for the post-stroke urinary incontinence during rehabilitation. Int. J. Rehabil. Res. 2020, 43, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Taly, A.B.; Srivastava, A.; Thyloth, M. Urodynamics post stroke in patients with urinary incontinence: Is there correlation between bladder type and site of lesion? Ann. Indian Acad. Neurol. 2009, 12, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A.; Falsini, C.; Martini, M.; Rossetti, M.A.; Verdesca, S.; Tosto, A. Urinary incontinence after ischemia stroke: Clinical and urodynamic studies. Neurol. Urodyn. 2014, 33, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, D.D.; Sidi, A.A.; Scott, A.B.; Pagel, J.M.; Goldish, G.D. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J. Urol. 1988, 139, 919–922. [Google Scholar] [CrossRef]
- Aoki, K.R.; Guyer, B. Botulinum toxin type A and other botulinum toxin serotypes: A comparative review of biochemical and pharmacological actions. Eur. J. Neurol. 2001, 8 (Suppl. S5), 21–29. [Google Scholar] [CrossRef]
- Anandan, C.; Jankovic, J. Botulinum toxin in movement disorder: An update. Toxins 2021, 13, 42. [Google Scholar] [CrossRef]
- Francisco, G.E.; Balbert, A.; Bavikatte, G.; Bensmail, D.; Carda, S.; Deltombe, T.; Draulans, N.; Escaldi, S.; Gross, R.; Jacinto, J.; et al. A practical guide to optimizing the benefits of post-stroke spasticity interventions with botulinum toxin A: An international group consensus. J. Rehabil. Med. 2021, 53, 2715. [Google Scholar] [CrossRef]
- Jocson, A.; Lew, M. Use of botulinum toxin in Parkinson’s disease. Park. Relat. Disord. 2019, 59, 57–64. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Liao, C.C.; Kuo, H.C. Current and potential urological applications of botulinum toxin A. Nat. Rev. Urol. 2015, 12, 519–533. [Google Scholar] [CrossRef]
- Cruz, F. Targets for botulinum toxin in the lower urinary tract. Neurourol. Urodyn. 2014, 33, 31–38. [Google Scholar] [CrossRef]
- Apostolidis, A.; Popat, R.; Yiangou, Y.; Cockayne, D.; Ford, A.P.D.W.; Davis, J.B.; Dasgupta, P.; Fowler, C.J.; Anand, P. Decreased sensory receptor P2X3 and TRPV1 in suburothelial nerve fibers following intradetrsuor injections of botulinum toxin for human detrusor overactivity. J. Urol. 2005, 174, 977–983. [Google Scholar] [CrossRef]
- Khera, M.; Somogyi, G.T.; Kiss, S.; Boone, T.B.; Smith, C.P. Botulinum toxin A inhibits ATP release from the bladder urothelium after chronic spinal cord injury. Neurochem. Int. 2004, 45, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Rapp, D.E.; Turk, K.W.; Bales, G.T.; Cook, S.P. Botulinum toxin type a inhibits calcitonin gene-related peptide release from isolated rat bladder. J. Urol. 2006, 175, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Lucioni, A.; Bales, G.T.; Lotan, T.L.; McGehee, D.S.; Cook, S.P.; Rapp, D.E. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008, 101, 366–370. [Google Scholar] [CrossRef] [PubMed]
- US Food & Drug Administration. BOTOX Label. Highlights of Prescribing Information. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103000s5307lbl.pdf (accessed on 10 February 2023).
- Wohlfarth, K.; Schwandt, I.; Wegner, F.; Jürgens, T.; Gelbrich, G.; Wagner, A.; Bogdahn, U.; Schulte-Mattler, W. Biological activity of two botulinum toxin type A complex (Dysport and Botox) in volunteers: A double-blind, randomized, dose-ranging study. J. Neurol. 2008, 255, 1932–1939. [Google Scholar] [CrossRef]
- Kuo, H.C. Comparison of effectiveness of detrusor, suburothelial and bladder base injections of botulinum toxin a for idiopathic detrusor overactivity. J. Urol. 2007, 178, 1359–1363. [Google Scholar] [CrossRef]
- Karsenty, G.; Elzayat, E.; Delapparent, T.; St-Denis, B.; Lemieux, M.C.; Corcos, J. Botulinum toxin type a injections into the trigone to treat idiopathic overactive bladder do not induce vesicoureteral reflux. J. Urol. 2007, 177, 1011–1014. [Google Scholar] [CrossRef]
- Mascarenhas, F.; Cocuzza, M.; Gomes, C.M.; Leao, N. Trigonal injection of botulinum toxin-A does not cause vesicoureteral reflux in neurogenic patients. Neurourol. Urodyn. 2008, 27, 311–314. [Google Scholar] [CrossRef]
- Giannantoni, A.; Costantini, E.; Di Stasi, S.M.; Tascini, M.C.; Bini, V.; Porena, M. Botulinum A toxin intravesical injections in the treatment of painful bladder syndrome: A pilot study. Eur. Urol. 2006, 49, 704–709. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.C. Effectiveness of urethral injection of botulinum A toxin in the treatment of voiding dysfunction after radical hysterectomy. Urol. Int. 2005, 75, 247–251. [Google Scholar] [CrossRef]
- Chen, J.L.; Chen, C.Y.; Kuo, H.C. Botulinum toxin A injection to the bladder neck and urethra for medically refractory lower urinary tract symptoms in men without prostatic obstruction. J. Formos. Med. Assoc. 2009, 108, 950–956. [Google Scholar] [CrossRef] [Green Version]
- Giannantoni, A.; Rossi, A.; Mearini, E.; Del Zingaro, M.; Porena, M.; Berardelli, A. Botulinum toxin A for overactive bladder and detrusor muscle overactivity in patients with Parkinson’s disease and multiple system atrophy. J. Urol. 2009, 182, 1453–1457. [Google Scholar] [CrossRef]
- Giannantoni, A.; Conte, A.; Proietti, S.; Giovannozzi, S.; Rossi, A.; Fabbrini, G.; Porena, M.; Berardelli, A. Botulinum toxin type A in patients with Parkinson’s disease and refractory overactive bladder. J. Urol. 2011, 186, 960–964. [Google Scholar] [CrossRef]
- Kulaksizoglu, H.; Parman, Y. Use of botulinum toxin-A for the treatment of overactive bladder symptoms in patients with Parkinson’s disease. Park. Relat. Disord. 2010, 16, 531–534. [Google Scholar] [CrossRef]
- Anderson, R.U.; Orenberg, E.K.; Glowe, P. OnabotulinumtoxinA office treatment for neurogenic bladder incontinence in Parkinson’s disease. Urology 2014, 83, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Vurture, G.; Peyronnet, B.; Feigin, A.; Biagioni, M.C.; Gilbert, R.; Rosenblum, N.; Frucht, S.; Di Rocco, A.; Nitti, V.W.; Brucker, B.M. Outcomes of intradetrusor onabotulinum toxin A injection in patients with Parkinson’s disease. Neurourol. Urodyn. 2018, 37, 2669–2677. [Google Scholar] [CrossRef]
- Atamian, A.; Sichez, P.C.; Michel, F.; Bandelier, Q.; Fall, M.; Gaillet, S.; Azoulay, J.P.; Lechevallier, E.; Karsenty, G. Intradetrusor injections of botulinum toxin A to treat urinary incontinence due to bladder overactivity during idiopathic Parkinson’s disease. Prog. Urol. 2021, 31, 430–438. [Google Scholar] [CrossRef]
- Mehta, S.; Hill, D.; Foley, N.; Hsieh, J.; Ethans, K.; Potter, P.; Baverstock, R.; Teasell, R.W.; Wolfe, D.; Spinal Cord Injury Rehabilitation Evidence Research Team. A meta-analysis of botulinum toxin sphincter injections in the treatment of incomplete voiding after spinal cord injury. Arch. Phys. Med. Rehabil. 2012, 93, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Satisfaction with urethral injection of botulinum toxin A for detrusor sphincter dyssynergia in patients with spinal cord lesion. Neurourol. Urodyn. 2008, 27, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Therapeutic outcome and quality of life between urethral and detrusor botulinum toxin treatment for patients with spinal cord lesions and detrusor sphincter dyssynergia. Int. J. Clin. 2013, 67, 1044–1049. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Chen, S.F.; Jhang, J.F.; Kuo, H.C. Therapeutic effect of urethral sphincter onabotulinumtoxinA injection for urethral sphincter hyperactivity. Neurourol. Urodyn. 2018, 37, 2651–2657. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Kuo, H.C. Therapeutic effects of botulinum toxin A, via urethral sphincter injection on voiding dysfunction due to different bladder and urethral sphincter dysfunctions. Toxins 2019, 11, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, L.H.; Coupe, J.; Cross, L.D.; Tan, A.L.; Watkins, C.L. Interventions for treating urinary incontinence after stroke in adults. Cochrane Database Syst. Rev. 2019, 2, CD004462. [Google Scholar] [PubMed]
- Shogenji, M.; Yoshida, M.; Sumiya, K.; Shimada, T.; Ikenaga, Y.; Ogawa, Y.; Hirako, K.; Sai, Y. Association of a continuous continence self-management program with independence in voiding behavior among stroke patients: A retrospective cohort study. Neurourol. Urodyn. 2022, 41, 1109–1120. [Google Scholar] [CrossRef]
- Vasudeva, P.; Kumar, A.; Yadav, S.; Kumar, N.; Chaudhry, N.; Prasad, V.; Nagendra Rao, S.; Yadav, P.; Patel, S. Neurological safety and efficacy of darifenacin and mirabegron for the treatment of overactive bladder in patients with history of cerebrovascular accident. Neurourol. Urodyn. 2021, 40, 2041–2047. [Google Scholar] [CrossRef]
- Lee, C.; Pizarro-Berdichevsky, J.; Clifton, M.M.; Vasavada, S.P. Sacral neuromodulation implant infection: Risk factor and prevention. Curr. Urol. Rep. 2017, 18, 16. [Google Scholar] [CrossRef]
- Tutoto, M.; Ammirati, E.; Van der Aa, F. What is new in meuromodulation for overactive bladder? Eur. Urol. Focus 2018, 4, 49–53. [Google Scholar] [CrossRef]
- Kuo, H.C. Therapeutic effects of suburothelial injection of botulinum A toxin for neurogenic detrusor overactivity due to chronic cerebrovascular accident and spinal cord lesions. Urology 2006, 67, 232–236. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Liao, C.H.; Tang, D.L.; Kuo, H.C. Efficacy and safety of intravesical onabotulinumtoxinA injection on elderly patients with chronic central nervous system lesions and overactive bladder. PLoS ONE 2014, 9, e105989. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Kuo, H.C. Botulinum A toxin treatment of urethral sphincter pseudodyssynergia in patients with cerebrovascular accidents or intracranial lesions. Urol. Int. 2004, 73, 156–161. [Google Scholar] [CrossRef]
- Knüpfer, S.C.; Schneider, S.A.; Averhoff, M.M.; Naumann, C.M.; Deuschl, G.; Jünemann, K.P.; Hamann, M.F. Preserved micturition after intradetrusor onabotulinumtoxinA injection for treatment of neurogenic bladder dysfunction in Parkinson’s disease. BMC Urol. 2016, 16, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Etiology | Author (Year) | Group/ Botulinum Brand | Patient Number (Male/Female) | Mean Age (SD) | Mean Duration of Disease (SD) | Injection Site | Dosage (Sites) | Anesthesia | Post-Injection Outcome | Adverse Events (Events/All Cases) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urodynamics | Clinical Outcome | Response Rate | ||||||||||
| PD | Giannantoni (2009) [53] | Botox | 4 (0/4) | 76.3 (4.8) | 9 years (3.5) | Detrusor (Trigone including) | 200 U in saline 20 mL (20 sites) | IVGA | IDC volume: Significantly increased (+234 mL) at 3rd month CMG capacity: Significantly increased (+225.5 mL) at 3rd month Pdet.Qmax: Significantly reduced (−3.5 cm H2O) at 3rd month Qmax: Significant reduced (−11.3 mL/s) at 3rd month PVR: Significantly increased (+88.8 mL) at 3rd month | Significantly improved daytime and nighttime Frequency Completely resolved daily UI (100%) Significantly improved I-QOL(+52.5) | 4/4 (100%) | 0/4 (0%) UTI 3/4 (75%) Dysuria 3/4 (75%) Voiding difficulty |
| Kulaksizoglu (2010) [55] | Dysport | 16 | 67.2 (5.1) | 6 years | Detrusor | 500 U in saline 30 mL (30 sites) | LA | CMG capacity: Significantly increased (+136 mL at 3rd month,+180 mL at 6th month, +183 mL at 9th month, +76.5 mL at 12th month) Pdet at IDC: Reduced (−28 cm H2O in men, −12 cm H2O in women) Persistent urodynamic UI: None after injection | Significantly reduced SEAPI score at 3rd, 6th, 9th and 12th month 6 incontinent patients at baseline: all with reduced UUI episode | 16/16 (100%) | 0/20 (0%) AUR need catheterization | |
| Giannantoni (2011) [54] | Botox | 8 (1/7) | 66 (3) | NA | Detrusor (Trigone including) | 100 U in saline 10 mL (10 sites) | IVGA | Complete resolution of IDC: 3/8 (37.5%) CMG capacity: Significantly increased at 1st, 3rd, and 6th month PVR: increased at 1st month, markedly decreased at 3rd and 6th month Qmax: No significant change Pdet.Qmax: No significant change | Significantly decreased frequency (daytime and nighttime) and UI Significantly improved I-QOL (+43) and VAS (+3.5) at 6th month | NA | 2/8 (25%) AUR need catheterization 0/8 (0%) UTI | |
| Jiang (2014) [70] | Botox | 9 | 73.6 (11.2) | NA | Detrusor | 100 U in saline 10 mL (20 sites) | IVGA | CMG capacity: Increased (+17 mL at 3rd month) Qmax: No significant change Pdet.Qmax: No significant change PVR: Significantly increased (+77.3 mL at 3rd month) | Significantly improved USS (−1.28) Improved urgency (−13 times) Improved UUI (−1.1 times) | NA | 1/9(11.1%) AUR need catheterization 3/9(33.3%) PVR > 150 mL 1/9(11.1%) Voiding difficulty need strain 1/9(11.1%) Hematuria 2/9(22.2%) UTI | |
| Anderson (2014) [56] | Botox | 20(12/8) | 70.4 | 10.6 years | Detrusor(Trigone including) | 100 U in saline 10 mL (10–20 sites) | LA | VV: No significant change at 1st, 3rd, and 6th month Qmax: Significant reduced (−4.7 mL/s) at 1st month, but not in 3rd and 6th month PVR: Significantly increased at 1st month (+106 mL) and 3rd month(+40 mL), but not in 6th month | Significantly improved UUI at 1st, 3rd, and 6th month Significantly improved AUA symptom scores at 1st, 3rd, and 6th month | 20/20 (100%) | 0/20 (0%) AUR need catheterization 2/20 (10%) UTI 0/20 (0%) Significant hematuria | |
| Knüpfer (2016) [72] | Botox | 10 (6/4) | 67.9 (5.4) | 9.2 years (8.2) | Detrusor (Trigone including) | 200 U in saline 20 mL (20 sites) | NA | CMG capacity: Significantly increased (+136 mL) Pdet.Max at voiding: Significantly reduced (−40 cm H2O) Compliance: Increased (+11.1 mL/cm H2O) VV: Significantly increased (+115 mL) PVR: scantly increased (+16 mL) Urodynamic DO: Markedly reduced (90% to 20%) Qmax: No significant change | Significantly improved frequency, nocturia and daily pad Significantly improved ICIQ score | 10/10 (100%) | 0/10 (0%) AUR need catheterization 0/10 (0%) UTI 0/10 (0%) Hematuria | |
| Vurture (2018) [57] | Botox | 24 (17/7) | 77.2 (7.5) | 9.8 years (5.7) | Detrusor (Trigone including) | 100 U in saline 10 mL (20 sites) | LA | PVR: Significantly increased (+108 mL) | Significantly decreased daily pad amount and UUI | 19/24 (79.2%) | 3/24 (12.5%) AUR need catheterization 6/24 (25%) UTI | |
| CVA | Chen (2004) [71] | Botox | 11 (5/6) | 66.5 (14.7) | NA | Sphincter | 100 U in saline 4 mL (4 sites) | IVGA | Pdet.Qmax: Significantly reduced (−24 cmH2O) Qmax: Significantly increased (+3.1 mL/s) | Significantly improved IPSS and QoL index in Botox group IPSS −13.6 in Botox vs. −4 in Control QoL Index −2.4 in Botox vs. −1.2 in Control | 10/11 (91%) | 0/11 (0%) |
| Control | 10 | 65.4 (15.5) | None | None | None | NA | NA | 4/10 (40%) | 0/10 (0%) | |||
| Kuo (2006) [69] | Botox | 12 (6/6) | 72.4 (5.7) | NA | Detrusor | 200 U in saline 20 mL (40 sites) | IVGA | IDC volume: Significantly Increased at 1st month (+139.9 cm H2O) but not at 3rd month (+56.3 mL) CMG capacity: Significantly increased at 1st month (+144.9 mL) but not at 3rd month (+56.2 mL) Pdet.Max at voiding: Reduced (−5.4 cm H2O at 1st month and −7.5 Cm H2O at 3rd month) PVR: Significantly increased at 1st month (+123 mL) but not at 3rd month (+31.5 mL) | Improved incontinence grade (−1.3 at 1st month and −0.9 at 3rd month) Significantly increased grade of voiding difficulty (+1.5 at 1st month and +0.7 at 3rd month) 7/12 (58%) voiding difficulty | 6/12 (50%) | 3/12 (25%) AUR need catheterization 21% Mild hematuria 25% UTI | |
| Jiang (2014) [70] | Botox | 23 | 73.6 (7.5) | NA | Detrusor | 100 U in saline 10 mL (20 sites) | IVGA | CMG capacity: Significantly increased (+160 mL at 3rd month) Qmax: No significant change Pdet.Qmax: No significant change PVR: Significantly increased (+112.5 mL at 3rd month) | Improved USS (−0.57) Improved urgency (−8.3 times) Significantly improved UUI (−7.8 times) | NA | 4/23 (17.4%) AUR need catheterization 12/23 (52.2%) PVR > 150 mL 17/23 (73.9%) Voiding difficulty need strain 2/23 (8.7%) Hematuria 1/23 (4.3%) UTI | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.-C.; Hsu, L.-N.; Lee, W.-C.; Chuang, Y.-C.; Wang, H.-J. Role of Urological Botulinum Toxin-A Injection for Overactive Bladder and Voiding Dysfunction in Patients with Parkinson’s Disease or Post-Stroke. Toxins 2023, 15, 166. https://doi.org/10.3390/toxins15020166
Hu J-C, Hsu L-N, Lee W-C, Chuang Y-C, Wang H-J. Role of Urological Botulinum Toxin-A Injection for Overactive Bladder and Voiding Dysfunction in Patients with Parkinson’s Disease or Post-Stroke. Toxins. 2023; 15(2):166. https://doi.org/10.3390/toxins15020166
Chicago/Turabian StyleHu, Ju-Chuan, Lin-Nei Hsu, Wei-Chia Lee, Yao-Chi Chuang, and Hung-Jen Wang. 2023. "Role of Urological Botulinum Toxin-A Injection for Overactive Bladder and Voiding Dysfunction in Patients with Parkinson’s Disease or Post-Stroke" Toxins 15, no. 2: 166. https://doi.org/10.3390/toxins15020166
APA StyleHu, J. -C., Hsu, L. -N., Lee, W. -C., Chuang, Y. -C., & Wang, H. -J. (2023). Role of Urological Botulinum Toxin-A Injection for Overactive Bladder and Voiding Dysfunction in Patients with Parkinson’s Disease or Post-Stroke. Toxins, 15(2), 166. https://doi.org/10.3390/toxins15020166