Aluminosilicates as a Double-Edged Sword: Adsorption of Aflatoxin B1 and Sequestration of Essential Trace Minerals in an In Vitro Gastrointestinal Poultry Model
Abstract
:1. Introduction
2. Results
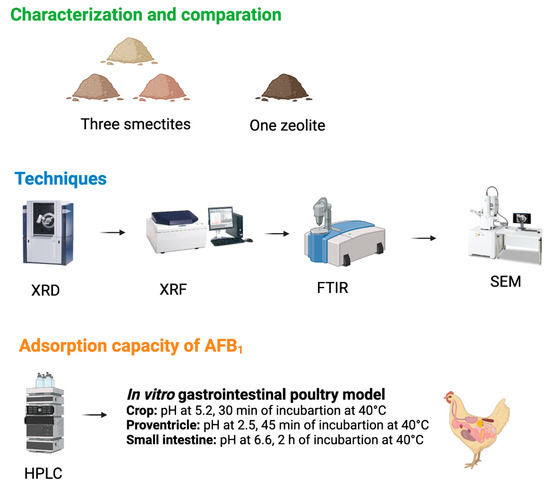
2.1. Characterization of Clay
2.2. Determination of CEC
2.3. Quantification of AFB1 in an In Vitro Poultry Gastrointestinal Model Using UPLC
2.4. Trace Inorganic Nutrient Adsorption in an In Vitro Poultry Gastrointestinal Model
3. Discussion
3.1. Characterization of Clay
3.2. CEC Values
3.3. Quantification of AFB1 Adsorption Capacity
3.4. Trace Inorganic Nutrient Adsorption
4. Conclusions
5. Materials and Methods
5.1. Reagents
5.2. Characterization of Clays
5.3. Determination of Cation Exchange Capacity (CEC)
5.4. Measurement of AFB1 Adsorption Capacity by Ultra Performance Liquid Chromatography (UPLC) Using an In Vitro Poultry Gastrointestinal Model
5.5. Measurement of Trace Inorganic Nutrient Adsorption Rate
5.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pathak, H.; Bhadauria, S.; Sudan, J. Aflatoxin Contamination in Food Crops: Causes, Detection, and Management: A Review. Food Prod. Process Nutr. 2021, 3, 17. [Google Scholar] [CrossRef]
- Diella, G.; Caggiano, G.; Ferrieri, F. Aflatoxin Contamination in Nuts Marketed in Italy: Preliminary Results. Ann. Lgiene Med. Prev. Comun. 2018, 30, 401–409. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; et al. Risk Assessment of Aflatoxins in Food. EFS2 2020, 18, e06040. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 07, 2170. [Google Scholar] [CrossRef]
- Dhakal, A.; Sbar, E. Aflatoxin Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. IJERPH 2020, 17, 423. [Google Scholar] [CrossRef]
- Bbosa, G.S.; Kitya, D.; Odda, J.; Ogwal-Okeng, J. Aflatoxins Metabolism, Effects on Epigenetic Mechanisms and Their Role in Carcinogenesis. Health 2013, 5, 14–34. [Google Scholar] [CrossRef]
- Jurišić, N.; Schwartz-Zimmermann, H.E.; Kunz-Vekiru, E.; Moll, W.D.; Schweiger, W.; Fowler, J.; Berthiller, F. Determination of Aflatoxin Biomarkers in Excreta and Ileal Content of Chickens. Poult. Sci. 2019, 98, 5551–5561. [Google Scholar] [CrossRef]
- Srinivasan, B.; Ghosh, S.; Webb, P.; Griswold, S.P.; Xue, K.S.; Wang, J.-S.; Mehta, S. Assessing an Aflatoxin Exposure Biomarker: Exploring the Interchangeability and Correlation between Venous and Capillary Blood Samples. Environ. Res. 2022, 215, 114396. [Google Scholar] [CrossRef]
- Some Traditional Herbal Medicines, Some Mycotoxins, Naphtalene and Styrene; Centre International de Recherche Sur le Cancer (Ed.) IARC monographs on the evaluation of carcinogenic risks tu humans; World Health Organization: Lyon, France, 2002; ISBN 978-92-832-1282-9. [Google Scholar]
- Kimanya, M.E.; Routledge, M.N.; Mpolya, E.; Ezekiel, C.N.; Shirima, C.P.; Gong, Y.Y. Estimating the Risk of Aflatoxin-Induced Liver Cancer in Tanzania Based on Biomarker Data. PLoS ONE 2021, 16, e0247281. [Google Scholar] [CrossRef]
- Guidance for FDA Action Levels for Aflatoxins in Animal Food 2019. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-683100-action-levels-aflatoxins-animal-feeds (accessed on 18 June 2023).
- Dhanasekaran, D.; Shanmugapriya, S.; Thajuddin, N.; Panneerselvam, A. Aflatoxins and Aflatoxicosis in Human and Animals. In Aflatoxins—Biochemistry and Molecular Biology; Guevara-Gonzalez, R.G., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-395-8. [Google Scholar]
- Singh, J.; Mehta, A. Rapid and Sensitive Detection of Mycotoxins by Advanced and Emerging Analytical Methods: A Review. Food Sci. Nutr. 2020, 8, 2183–2204. [Google Scholar] [CrossRef]
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for Detection of Aflatoxins in Agricultural Food Crops. J. Appl. Chem. 2014, 2014, 706291. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, S.S.; Chhillar, A.K.; Rana, J.S. An Overview of Nanomaterial Based Biosensors for Detection of Aflatoxin B1 Toxicity in Foods. Food Chem. Toxicol. 2021, 152, 112201. [Google Scholar] [CrossRef]
- Fouad, A.; Ruan, D.; El-Senousey, H.; Chen, W.; Jiang, S.; Zheng, C. Harmful Effects and Control Strategies of Aflatoxin B1 Produced by Aspergillus Flavus and Aspergillus Parasiticus Strains on Poultry: Review. Toxins 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Monson, M.; Coulombe, R.; Reed, K. Aflatoxicosis: Lessons from Toxicity and Responses to Aflatoxin B1 in Poultry. Agriculture 2015, 5, 742–777. [Google Scholar] [CrossRef]
- Asghar, M.A.; Ahmed, A.; Asghar, M.A. Influence of Temperature and Environmental Conditions on Aflatoxin Contamination in Maize Collected from Different Regions of Pakistan during 2016–2019. J. Stored Prod. Res. 2020, 88, 101637. [Google Scholar] [CrossRef]
- Kumar, P.; Gupta, A.; Mahato, D.K.; Pandhi, S.; Pandey, A.K.; Kargwal, R.; Mishra, S.; Suhag, R.; Sharma, N.; Saurabh, V.; et al. Aflatoxins in Cereals and Cereal-Based Products: Occurrence, Toxicity, Impact on Human Health, and Their Detoxification and Management Strategies. Toxins 2022, 14, 687. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of Food Processing and Detoxification Treatments on Mycotoxin Contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Sipos, P.; Peles, F.; Brassó, D.L.; Béri, B.; Pusztahelyi, T.; Pócsi, I.; Győri, Z. Physical and Chemical Methods for Reduction in Aflatoxin Content of Feed and Food. Toxins 2021, 13, 204. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Progress on the Detoxification of Aflatoxin B1 Using Natural Anti-Oxidants. Food Chem. Toxicol. 2022, 169, 113417. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Zhou, X.; Liu, M.; Zang, H.; Liu, X.; Shan, A.; Feng, X. Alleviation of Oral Exposure to Aflatoxin B1-Induced Renal Dysfunction, Oxidative Stress, and Cell Apoptosis in Mice Kidney by Curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Omur, A. Activity of resveratrol on the influence of aflatoxin B1 on the testes of Sprague dawley rats. Pol. J. Vet. Sci. 2023, 22, 313–320. [Google Scholar] [CrossRef]
- Wu, J.; Gan, Z.; Zhuo, R.; Zhang, L.; Wang, T.; Zhong, X. Resveratrol Attenuates Aflatoxin B1-Induced ROS Formation and Increase of M6A RNA Methylation. Animals 2020, 10, 677. [Google Scholar] [CrossRef]
- Okun, D.O.; Khamis, F.M.; Muluvi, G.M.; Ngeranwa, J.J.; Ombura, F.O.; Yongo, M.O.; Kenya, E.U. Distribution of Indigenous Strains of Atoxigenic and Toxigenic Aspergillus Flavus and Aspergillus Parasiticus in Maize and Peanuts Agro-Ecological Zones of Kenya. Agric. Food Secur. 2015, 4, 14. [Google Scholar] [CrossRef]
- Maxwell, L.A.; Callicott, K.A.; Bandyopadhyay, R.; Mehl, H.L.; Orbach, M.J.; Cotty, P.J. Degradation of Aflatoxins B1 by Atoxigenic Aspergillus flavus Biocontrol Agents. Plant Dis. 2021, 105, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Damato, A.; Vianello, F.; Novelli, E.; Balzan, S.; Gianesella, M.; Giaretta, E.; Gabai, G. Comprehensive Review on the Interactions of Clay Minerals With Animal Physiology and Production. Front. Vet. Sci. 2022, 9, 889612. [Google Scholar] [CrossRef] [PubMed]
- Nadziakiewicza, M.; Kehoe, S.; Micek, P. Physico-Chemical Properties of Clay Minerals and Their Use as a Health Promoting Feed Additive. Animals 2019, 9, 714. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Structure and Mineralogy of Clay Minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 21–81. ISBN 978-0-08-099364-5. [Google Scholar]
- Odom, I.E. Smectite Clay Minerals: Properties and Uses. Phil. Trans. R. Soc. Lond. A 1984, 311, 391–409. [Google Scholar] [CrossRef]
- Teich-McGoldrick, S.L.; Greathouse, J.A.; Jové-Colón, C.F.; Cygan, R.T. Swelling Properties of Montmorillonite and Beidellite Clay Minerals from Molecular Simulation: Comparison of Temperature, Interlayer Cation, and Charge Location Effects. J. Phys. Chem. C 2015, 119, 20880–20891. [Google Scholar] [CrossRef]
- García-Romero, E.; María Manchado, E.; Suárez, M.; García-Rivas, J. Spanish Bentonites: A Review and New Data on Their Geology, Mineralogy, and Crystal Chemistry. Minerals 2019, 9, 696. [Google Scholar] [CrossRef]
- García-Romero, E.; Lorenzo, A.; García-Vicente, A.; Morales, J.; García-Rivas, J.; Suárez, M. On the Structural Formula of Smectites: A Review and New Data on the Influence of Exchangeable Cations. J. Appl. Crystallogr. 2021, 54, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Britannica. The Editors of Encyclopaedia Tectosilicate. Encyclopedia Britannica 2018. Available online: https://www.britannica.com/science/tectosilicate (accessed on 18 June 2023).
- Yamada, H.; Tamura, K.; Watanabe, Y.; Iyi, N.; Morimoto, K. Geomaterials: Their Application to Environmental Remediation. Sci. Technol. Adv. Mater. 2011, 12, 064705. [Google Scholar] [CrossRef] [PubMed]
- Gougazeh, M.; Buhl, J.-C. Synthesis and Characterization of Zeolite A by Hydrothermal Transformation of Natural Jordanian Kaolin. J. Assoc. Arab. Univ. Basic Appl. Sci. 2014, 15, 35–42. [Google Scholar] [CrossRef]
- Stylianou, M.; Inglezakis, V.; Agapiou, A.; Itskos, G.; Jetybayeva, A.; Loizidou, M. A Comparative Study on Phyllosilicate and Tectosillicate Mineral Structural Properties. DWT 2018, 112, 119–146. [Google Scholar] [CrossRef]
- Kraljević Pavelić, S.; Simović Medica, J.; Gumbarević, D.; Filošević, A.; Pržulj, N.; Pavelić, K. Critical Review on Zeolite Clinoptilolite Safety and Medical Applications In Vivo. Front. Pharmacol. 2018, 9, 1350. [Google Scholar] [CrossRef]
- Prvulovic, D.; Kojic, D.; Grubor-Lajsic, G.; Kosarcic, S. The Effects of Dietary Inclusion of Hydrated Aluminosilicate on Performance and Biochemical Parameters of Broiler Chickens. Turk. J. Vet. Anim. Sci. 2008, 32, 159–166. [Google Scholar]
- Safaeikatouli, M.; Boldaji, F.; Dastar, B.; Hassani, S. The Effect of Dietary Silicate Minerals Supplementation on Apparent Ileal Digestibility of Energy and Protein in Broiler Chickens. Int. J. Agric. Biol. 2012, 299–302. [Google Scholar]
- Alharthi, A.S.; Al Sulaiman, A.R.; Aljumaah, R.S.; Alabdullatif, A.A.; Ferronato, G.; Alqhtani, A.H.; Al-Garadi, M.A.; Al-sornokh, H.; Abudabos, A.M. The Efficacy of Bentonite and Zeolite in Reducing Aflatoxin B1 Toxicity on Production Performance and Intestinal and Hepatic Health of Broiler Chickens. Ital. J. Anim. Sci. 2022, 21, 1181–1189. [Google Scholar] [CrossRef]
- Andrejkovicová, S.; Madejová, J.; Czímerowá, A.; Galko, I.; Dohrmann, R.; Komadel, P. Mineralogy and Chemistry of Fe-Richbentonite from the Lieskovec Deposit. Geol. Carpath. 2006, 57, 371–378. [Google Scholar]
- Danková, Z.; Mockovčiaková, A.; Dolinská, S. Influence of Ultrasound Irradiation on Cadmium Cations Adsorption by Montmorillonite. Desalination Water Treat. 2014, 52, 5462–5469. [Google Scholar] [CrossRef]
- Krivacsy, Z.; Hlavay, J. A Simple Method for the Determination of Clinoptilolite in Natural Zeolite Rocks. Zeolites 1995, 15, 551–555. [Google Scholar] [CrossRef]
- Król, M.; Koleżyński, A.; Mozgawa, W. Vibrational Spectra of Zeolite Y as a Function of Ion Exchange. Molecules 2021, 26, 342. [Google Scholar] [CrossRef] [PubMed]
- Desheng, Q.; Fan, L.; Yanhu, Y.; Niya, Z. Adsorption of Aflatoxin B1 on Montmorillonite. Poult. Sci. 2005, 84, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tong, D.; Yu, W. Smectite Nanomaterials: Preparation, Properties, and Functional Applications. In Nanomaterials from Clay Minerals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 335–364. ISBN 978-0-12-814533-3. [Google Scholar]
- Dakovic, A.; Tomasevic-Canovic, M.; Dondur, V.; Vujakovic, A.; Radosevic, P. Kinetics of Aflatoxin B1 and G2 Adsorption on Ca-Clinoptilolite. J. Serbian Chem. Soc. 2000, 65, 715–723. [Google Scholar] [CrossRef]
- Znak, Z.; Zin, O.; Mashtaler, A.; Korniy, S.; Sukhatskiy, Y.; Gogate, P.R.; Mnykh, R.; Thanekar, P. Improved Modification of Clinoptilolite with Silver Using Ultrasonic Radiation. Ultrason. Sonochemistry 2021, 73, 105496. [Google Scholar] [CrossRef] [PubMed]
- Grim, R.E.; Kodama, H. Clay Mineral. Encyclopaedia Britannica. 2022. Available online: https://www.britannica.com/science/clay-mineral (accessed on 18 June 2023).
- Karnland, O. Chemical and Mineralogical Characterization of the Bentonite Buffer for the Acceptance Control Procedure in a KBS-3 Repository. 2010. Available online: https://www.osti.gov/etdeweb/biblio/1032587 (accessed on 18 June 2023).
- Anjos, F.R.D.; Ledoux, D.R.; Rottinghaus, G.E.; Chimonyo, M. Efficacy of Mozambican Bentonite and Diatomaceous Earth in Reducing the Toxic Effects of Aflatoxins in Chicks. World Mycotoxin J. 2016, 9, 63–72. [Google Scholar] [CrossRef]
- Stankovic, N.; Logar, M.; Lukovic, J.; Pantic, J.; Miljevic, M.; Babic, B.; Radosavljevic-Mihajlovic, A. Characterization of Bentonite Clay from “Greda” Deposit. PAC 2011, 5, 97–101. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, A.; Pérez, R. Kinetic Adsorption Study of Silver Nanoparticles on Natural Zeolite: Experimental and Theoretical Models. Appl. Sci. 2015, 5, 1869–1881. [Google Scholar] [CrossRef]
- Damian, G.; Damian, F.; Szakács, Z.; Iepure, G.; Aştefanei, D. Mineralogical and Physico-Chemical Characterization of the Oraşu-Nou (Romania) Bentonite Resources. Minerals 2021, 11, 938. [Google Scholar] [CrossRef]
- Adikary, S.U.; Ashokcline, M.; Nirojan, K. Characterization of Montmorillonite Clay from Naturally Occurring Clay Deposits in Murunkan Area. 2015. Available online: http://ir.kdu.ac.lk/handle/345/1347 (accessed on 18 June 2023).
- Fil, B.A.; Özmetin, C.; Korkmaz, M. Characterization and Electrokinetic Properties of Montmorillonite. Bulg. Chem. Commun. 2014, 46, 258–263. [Google Scholar]
- Zhang, S.-S.; Yang, N.; Zhuang, X.; Ren, L.; Natarajan, V.; Cui, Z.; Si, H.; Xin, X.; Ni, S.-Q.; Zhan, J. Montmorillonite Immobilized Fe/Ni Bimetallic Prepared by Dry in-Situ Hydrogen Reduction for the Degradation of 4-Chlorophenlo. Sci. Rep. 2019, 9, 13388. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.S.; Shannon, E.V. The Minerals of Bentonite and Related Clays and Their Physical Properties. J. Am. Ceram. Soc. 1926, 9, 77–96. [Google Scholar] [CrossRef]
- Santos, H.S.; Cesio, A.M.; Gauna, M.; Justo, V.F.; Volzone, C. Structural Changes of Cr-Beidellite Treated up to 1350 °C in Oxygen or Nitrogen Atmospheres. Cerâmica 2018, 64, 64–68. [Google Scholar] [CrossRef]
- Kyziol-Komosinska, J.; Barba, F.; Callejas, P.; Rosik-Dulewska, C. Beidellite and Other Natural Low-Cost Sorbents to Remove Chromium and Cadmium from Water and Wastewater. Bol. Soc. Esp. Ceram. Vidr. 2010, 49, 121–128. [Google Scholar]
- Rashid, T.; Iqbal, D.; Hazafa, A.; Hussain, S.; Sher, F.; Sher, F. Formulation of Zeolite Supported Nano-Metallic Catalyst and Applications in Textile Effluent Treatment. J. Environ. Chem. Eng. 2020, 8, 104023. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, R.; Mukhiya, R.; Awasthi, K.; Kumar, M. Zinc-Oxide Based EGFET PH Sensors. In Nanostructured Zinc Oxide; Elsevier: Amsterdam, The Netherlands, 2021; pp. 459–481. ISBN 978-0-12-818900-9. [Google Scholar]
- Akpomie, K.G.; Dawodu, F.A. Acid-Modified Montmorillonite for Sorption of Heavy Metals from Automobile Effluent. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 1–12. [Google Scholar] [CrossRef]
- Dimowa, L.; Tzvetanova, Y. Powder XRD Study of Changes of Cd2+ Modified Clinoptilolite at Different Stages of the Ion Exchange Process. Minerals 2021, 11, 1130. [Google Scholar] [CrossRef]
- Ural, N. The Significance of Scanning Electron Microscopy (SEM) Analysis on the Microstructure of Improved Clay: An Overview. Open Geosci. 2021, 13, 197–218. [Google Scholar] [CrossRef]
- Belousov, P.; Semenkova, A.; Egorova, T.; Romanchuk, A.; Zakusin, S.; Dorzhieva, O.; Tyupina, E.; Izosimova, Y.; Tolpeshta, I.; Chernov, M.; et al. Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite and Diatomite. Minerals 2019, 9, 625. [Google Scholar] [CrossRef]
- De León, M.A.; Sergio, M.; Bussi, J.; Ortiz de la Plata, G.B.; Cassano, A.E.; Alfano, O.M. Application of a Montmorillonite Clay Modified with Iron in Photo-Fenton Process. Comparison with Goethite and NZVI. Environ. Sci. Pollut. Res. 2015, 22, 864–869. [Google Scholar] [CrossRef]
- Yin, X.; Xie, X.; Wu, X.; An, X. Catalytic Performance of Nickel Immobilized on Organically Modified Montmorillonite in the Steam Reforming of Ethanol for Hydrogen Production. J. Fuel Chem. Technol. 2016, 44, 689–697. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Kusunose, T.; Ioku, K.; Yamasaki, N.; Malla, P.B.; Komarneni, S. Hydrothermal Crystallization Mechanism of Sodium Beidellite from Amorphous Gel. J. Mater. Sci. Lett. 1995, 14, 1770–1772. [Google Scholar] [CrossRef]
- Kloprogge, J.T. Synthesis and Paragenesis of Na-Beidellite as a Function of Temperature, Water Pressure, and Sodium Activity. Clays Clay Miner. 1993, 41, 423–430. [Google Scholar] [CrossRef]
- Christidis, G.E. Assessment of Industrial Clays. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 425–449. ISBN 978-0-08-099364-5. [Google Scholar]
- Calabria, J.A.A.; Amaral, D.N.d.; Ladeira, A.C.Q.; Cota, S.D.S.; Silva, T.S.S. Determination of the Cation Exchange Capacity of Bentonite Exposed to Hyperalkaline Fluid. In Proceedings of the INAC 2013: International Nuclear Atlantic Conference, Recife, Brazil, 24–29 November 2013. [Google Scholar]
- Choo, K.Y.; Bai, K. The Effect of the Mineralogical Composition of Various Bentonites on CEC Values Determined by Three Different Analytical Methods. Appl. Clay Sci. 2016, 126, 153–159. [Google Scholar] [CrossRef]
- Rihayat, T.; Salim, S.; Arlina, A.; Fona, Z.; Jalal, R.; Alam, P.N.; Zaimahwati; Sami, M.; Syarif, J.; Juhan, N. Determination of CEC Value (Cation Exchange Capacity) of Bentonites from North Aceh and Bener Meriah, Aceh Province, Indonesia Using Three Methods. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012054. [Google Scholar] [CrossRef]
- Wiyantoko, B.; Rahmah, N. Measurement of Cation Exchange Capacity (CEC) on Natural Zeolite by Percolation Method. In Proceedings of the 2nd International Seminar on Chemical Education (ISCE) 2017, Yogyakarta, Indonesia, 12–13 September 2017; p. 020021. [Google Scholar]
- Durães, N.; Novo, L.A.B.; Candeias, C.; da Silva, E.F. Distribution, Transport and Fate of Pollutants. In Soil Pollution; Elsevier: Amsterdam, The Netherlands, 2018; pp. 29–57. ISBN 978-0-12-849873-6. [Google Scholar]
- Mason, J.A.; Zanner, C.W. GRASSLAND SOILS. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; pp. 138–145. ISBN 978-0-12-348530-4. [Google Scholar]
- Barrientos-Velázquez, A.L.; Marroquin Cardona, A.; Liu, L.; Phillips, T.; Deng, Y. Influence of Layer Charge Origin and Layer Charge Density of Smectites on Their Aflatoxin Adsorption. Appl. Clay Sci. 2016, 132–133, 281–289. [Google Scholar] [CrossRef]
- Wang, M.; Hearon, S.E.; Phillips, T.D. A High Capacity Bentonite Clay for the Sorption of Aflatoxins. Food Addit. Contam. Part A 2020, 37, 332–341. [Google Scholar] [CrossRef]
- Dvorák, M. [Ability of bentonite and natural zeolite to adsorb aflatoxin from liquid media]. Vet. Med. 1989, 34, 307–316. [Google Scholar]
- Nuryono, N.; Agus, A.; Wedhastri, S.; Maryudhani, Y.M.S.; Pranowo, D.; Yunianto, Y.; Razzazi-Fazeli, E. Adsorption of Aflatoxin B1 in Corn on Natural Zeolite and Bentonite. Indones. J. Chem. 2012, 12, 279–286. [Google Scholar] [CrossRef]
- Savari, M.; Dehghan Banadaki, M.; Rezayazdi, K.; Javan-Nikkhah, M. A Comparison of Mineral Adsorbents Zeolite and Bentonite vs. Organic Adsorbent (Mycosorb) and Mineral—Organic Adsorbent (Biotox) as Their RegardsTheir Potential of Adsorbing Aflatoxin B1. Iran. J. Anim. Sci. 2013, 44, 105–112. [Google Scholar] [CrossRef]
- Tomasevic-Canovic, M.; Dakovic, A.; Markovic, V.; Stojsic, D. The Effect of Exchangeable Cations in Clinoptilolite and Montmorillonite on the Adsorption of Aflatoxin B1. J. Serbian Chem. Soc. 2001, 66, 555–561. [Google Scholar] [CrossRef]
- Bočarov-Stančić, A.; Lopičić, Z.; Bodroža-Solarov, M.; Stanković, S.; Janković, S.; Milojković, J.; Krulj, J. In Vitro Removing of Mycotoxins by Using Different Inorganic Adsorbents and Organic Waste Materials from Serbia. Food Feed. Res. 2018, 45, 87–96. [Google Scholar] [CrossRef]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef]
- Ayo, E.; Matemu, A.; Laswai, G.; Kimanya, M. An In Vitro Evaluation of the Capacity of Local Tanzanian Crude Clay and Ash-Based Materials in Binding Aflatoxins in Solution. Toxins 2018, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, F.; Kuttappan, V.A.; Cook, K.; Buresh, B.; Roux, M.; Hancock, D.; Vázquez-Añón, M. Effect of Methionine and Trace Minerals (Zinc, Copper and Manganese) Supplementation on Growth Performance of Broilers Subjected to Eimeria Challenge. Front. Physiol. 2022, 13, 991320. [Google Scholar] [CrossRef]
- Chen, C.; Qu, M.; Liang, H.; Ouyang, K.; Xiong, Z.; Zheng, Y.; Yan, Q.; Xu, L. Gastrointestinal Digestibility Insights of Different Levels of Coated Complex Trace Minerals Supplementation on Growth Performance of Yellow-Feathered Broilers. Front. Vet. Sci. 2022, 9, 982699. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.D.; Zhao, J.; Harrell, R.J.; Atwell, C.A.; Dibner, J.J. Trace Mineral Nutrition in Poultry and Swine. Asian Australas. J. Anim. Sci 2010, 23, 1527–1534. [Google Scholar] [CrossRef]
- Schaible, P.J. The Minerals in Poultry Nutrition—A Review. Poult. Sci. 1941, 20, 278–288. [Google Scholar] [CrossRef]
- Goff, J.P. Invited Review: Mineral Absorption Mechanisms, Mineral Interactions That Affect Acid–Base and Antioxidant Status, and Diet Considerations to Improve Mineral Status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a Few Heavy Metals on Natural and Modified Kaolinite and Montmorillonite: A Review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Bish, D.L. Parallels and Distinctions Between Clay Minerals and Zeolites. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 783–800. ISBN 978-0-08-099364-5. [Google Scholar]
- Lin, S. Heavy Metal Removal from Water by Sorption Using Surfactant-Modified Montmorillonite. J. Hazard. Mater. 2002, 92, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Van Groeningen, N.; Glück, B.; Christl, I.; Kretzschmar, R. Surface Precipitation of Mn 2+ on Clay Minerals Enhances Cd 2+ Sorption under Anoxic Conditions. Environ. Sci. Process. Impacts 2020, 22, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.G.; Troeh, F.R. Introductory Soil Science: Laboratory Manual, 3rd ed.; Oxford University Press: New York, NY, USA, 1995; ISBN 978-0-19-509436-7. [Google Scholar]
- Solís-Cruz, B.; Hernández-Patlán, D.; Beyssac, E.; Latorre, J.; Hernandez-Velasco, X.; Merino-Guzman, R.; Tellez, G.; López-Arellano, R. Evaluation of Chitosan and Cellulosic Polymers as Binding Adsorbent Materials to Prevent Aflatoxin B1, Fumonisin B1, Ochratoxin, Trichothecene, Deoxynivalenol, and Zearalenone Mycotoxicoses Through an In Vitro Gastrointestinal Model for Poultry. Polymers 2017, 9, 529. [Google Scholar] [CrossRef] [PubMed]





| Clay Samples | Fe2O3 | TiO2 | CaO | K2O | SiO2 | Al2O3 | MgO | Na2O | SO3 |
|---|---|---|---|---|---|---|---|---|---|
| C-1 | 2.56 | 0.19 | 2.23 | 0.48 | 69.37 | 9.14 | 5.02 | 0.25 | 0.10 |
| C-2 | 2.31 | 0.20 | 1.40 | 1.21 | 72.71 | 11.07 | 2.64 | 1.91 | 1.05 |
| C-3 | 5.47 | 0.63 | 9.48 | 2.23 | 50.22 | 13.97 | 3.77 | 2.28 | 1.01 |
| C-4 | 2.25 | 0.28 | 2.96 | 4.99 | 65.79 | 13.33 | 1.32 | 0.46 | 0.20 |
| Clay Samples | Mineral | Percentage (%) | Chemical Formula |
|---|---|---|---|
| C-1 | Montmorillonite | 61.0 | Ca0.2(Al,Mg)2Si4O10(OH)2·4H2O |
| Cristobalite | 21.0 | SiO2 | |
| Beidellite | 10.0 | Na0.3Al2(Si,Al)4O10(OH)2·2H2O | |
| Orthoclase | 2.5 | KAlSi3O8 | |
| Quartz | 5.5 | SiO2 | |
| C-2 | Beidellite | 55.0 | Na0.3Al2(Si,Al)4O10(OH)2·2H2O |
| Cristobalite | 24.0 | SiO2 | |
| Orthoclase | 7.0 | KAlSi3O8 | |
| Quartz | 6.0 | SiO2 | |
| Albite | 3.5 | NaAlSi3O8 | |
| Clinoptilolite | 3.0 | (Na0.52K2.44Ca1.48)(Al6.59Si29.41O72)(H2O)28.64 | |
| Gypsum | 1.5 | CaSO4·2H2O | |
| C-3 | Beidellite | 36.5 | Na0.3Al2(Si,Al)4O10(OH)2·2H2O |
| Montmorillonite | 15.0 | Ca0.2(Al,Mg)2Si4O10(OH)2·4H2O | |
| Illite | 14.0 | K(Al4Si2O9)(OH)3) | |
| Calcite | 12.0 | CaCO3 | |
| Albite | 10.0 | NaAlSi3O8 | |
| Quartz | 7.0 | SiO2 | |
| Sanidine | 4.0 | KAlSi3O8 | |
| Gypsum | 1.5 | CaSO4·2H2O | |
| C-4 | Clinoptilolite | 80.0 | (Na0.52K2.44Ca1.48)(Al6.59Si29.41O72)(H2O)28.64 |
| Sanidine | 8.0 | KAlSi3O8 | |
| Montmorillonite | 5.0 | Na0.3(Al,Mg)2Si4O10(OH)2·4H2O | |
| Cristobalite | 4.0 | SiO2 | |
| Illite | 2.0 | K(Al4Si2O9)(OH)3) | |
| Quartz | 1.0 | SiO2 |
| Clay Samples | CEC (cmol(+)/kg) |
|---|---|
| C-1 | 57.44 |
| C-2 | 54.72 |
| C-3 | 60.18 |
| C-4 | 38.29 |
| Clay Samples | AFB1 Adsorbed (ng/kg) | AFB1 Adsorbed (%) |
|---|---|---|
| C-1 | 0.199 ± 0.00012 | 99.5 |
| C-2 | 0.184 ± 0.00013 | 92.02 |
| C-3 | 0.176 ± 0.00016 | 88.40 |
| C-4 | 0.161 ± 0.0004 | 80.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Martínez, S.P.; Delgado-Cedeño, A.; Ramos-Zayas, Y.; Franco-Molina, M.A.; Méndez-Zamora, G.; Marroquín-Cardona, A.G.; Kawas, J.R. Aluminosilicates as a Double-Edged Sword: Adsorption of Aflatoxin B1 and Sequestration of Essential Trace Minerals in an In Vitro Gastrointestinal Poultry Model. Toxins 2023, 15, 519. https://doi.org/10.3390/toxins15090519
Hernández-Martínez SP, Delgado-Cedeño A, Ramos-Zayas Y, Franco-Molina MA, Méndez-Zamora G, Marroquín-Cardona AG, Kawas JR. Aluminosilicates as a Double-Edged Sword: Adsorption of Aflatoxin B1 and Sequestration of Essential Trace Minerals in an In Vitro Gastrointestinal Poultry Model. Toxins. 2023; 15(9):519. https://doi.org/10.3390/toxins15090519
Chicago/Turabian StyleHernández-Martínez, Sara Paola, Armando Delgado-Cedeño, Yareellys Ramos-Zayas, Moisés Armides Franco-Molina, Gerardo Méndez-Zamora, Alicia Guadalupe Marroquín-Cardona, and Jorge R. Kawas. 2023. "Aluminosilicates as a Double-Edged Sword: Adsorption of Aflatoxin B1 and Sequestration of Essential Trace Minerals in an In Vitro Gastrointestinal Poultry Model" Toxins 15, no. 9: 519. https://doi.org/10.3390/toxins15090519
APA StyleHernández-Martínez, S. P., Delgado-Cedeño, A., Ramos-Zayas, Y., Franco-Molina, M. A., Méndez-Zamora, G., Marroquín-Cardona, A. G., & Kawas, J. R. (2023). Aluminosilicates as a Double-Edged Sword: Adsorption of Aflatoxin B1 and Sequestration of Essential Trace Minerals in an In Vitro Gastrointestinal Poultry Model. Toxins, 15(9), 519. https://doi.org/10.3390/toxins15090519