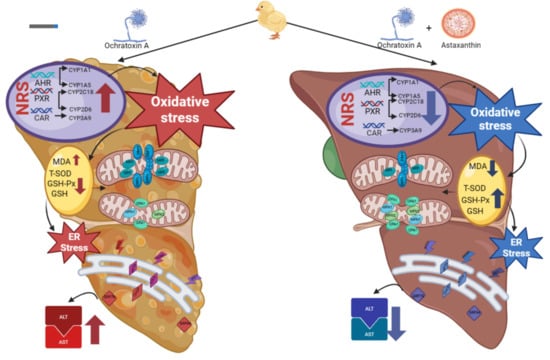
Protective Effects of Astaxanthin on Ochratoxin A-Induced Liver Injury: Effects of Endoplasmic Reticulum Stress and Mitochondrial Fission–Fusion Balance
Abstract
:1. Introduction
2. Results and Analysis
2.1. Intervention Effect of ASTA on OTA-Induced Liver Injury in Broilers
2.2. Effects of ASTA on OTA-Induced Liver Nuclear Receptors, Cytochrome Enzymes, and Oxidative Stress Indexes of Broilers
2.3. Observation of Broiler Liver Ultrastructure
2.4. Effects of ASTA on Liver ER Stress-Related Genes and Proteins in OTA-Induced Broilers
2.5. Effects of ASTA on OTA-Induced Expressions of Mitochondrial Dynamics-Related Genes and Proteins in Broilers
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Design of Animal Experiments
5.2. Determinations of Body Weight and Liver Index of Broilers
5.3. Determination of Serum Biochemical and Antioxidant Enzyme Indexes
5.4. Hematoxylin–Eosin (HE) Staining Analysis
5.5. Transmission Electron Microscopy
5.6. RNA Extraction and Real-Time Fluorescence Quantitative PCR
5.7. Western Blot
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.; Zhang, B.; Dai, Y.; Li, H.; Xu, W. A Review: Epigenetic Mechanism in Ochratoxin A Toxicity Studies. Toxins 2017, 9, 113. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D. Studies on carcinogenic and toxic effects of ochratoxin A in chicks. Toxins 2010, 2, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Abdel Halim, A.S.; Rudayni, H.A.; Chaudhary, A.A.; Ali, M.A.M. MicroRNAs: Small molecules with big impacts in liver injury. J. Cell. Physiol. 2023, 238, 32–69. [Google Scholar] [CrossRef] [PubMed]
- Tessari, P.; Vettore, M.; Millioni, R.; Puricelli, L.; Orlando, R. Effect of liver cirrhosis on phenylalanine and tyrosine metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hao, H.; Wang, H.; Guo, C.; Kang, A.; Wang, G. Reversing effects of lignans on CCl4-induced hepatic CYP450 down regulation by attenuating oxidative stress. J. Ethnopharmacol. 2014, 155, 213–221. [Google Scholar] [CrossRef]
- Li, P.; Li, K.; Zou, C.; Tong, C.; Sun, L.; Cao, Z.; Yang, S.; Lyu, Q. Selenium Yeast Alleviates Ochratoxin A-Induced Hepatotoxicity via Modulation of the PI3K/AKT and Nrf2/Keap1 Signaling Pathways in Chickens. Toxins 2020, 12, 143. [Google Scholar] [CrossRef]
- Anelli, T.; Sitia, R. Protein quality control in the early secretory pathway. EMBO J. 2008, 27, 315–327. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Vallée, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Detmer, S.A.; Chan, D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007, 8, 870–879. [Google Scholar] [CrossRef]
- Palmer, C.S.; Osellame, L.D.; Laine, D.; Koutsopoulos, O.S.; Frazier, A.E.; Ryan, M.T. MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep. 2011, 12, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Kalia, R.; Wang, R.Y.; Yusuf, A.; Thomas, P.V.; Agard, D.A.; Shaw, J.M.; Frost, A. Structural basis of mitochondrial receptor binding and constriction by DRP1. Nature 2018, 558, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Lackner, L.L.; West, M.; DiBenedetto, J.R.; Nunnari, J.; Voeltz, G.K. ER tubules mark sites of mitochondrial division. Science 2011, 334, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Yapa, N.M.B.; Lisnyak, V.; Reljic, B.; Ryan, M.T. Mitochondrial dynamics in health and disease. FEBS Lett. 2021, 595, 1184–1204. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Mihara, K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J. Biochem. 2011, 149, 241–251. [Google Scholar] [CrossRef]
- Rojo, M.; Legros, F.; Chateau, D.; Lombès, A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J. Cell Sci. 2002, 115 Pt 8, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Lamanilao, G.G.; Dogan, M.; Patel, P.S.; Azim, S.; Patel, D.S.; Bhattacharya, S.K.; Eason, J.D.; Kuscu, C.; Kuscu, C.; Bajwa, A. Key hepatoprotective roles of mitochondria in liver regeneration. Am. J. Physiol.—Gastrointest. Liver Physiol. 2023, 324, G207–G218. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Song, R.; Xu, Y.; Liu, X.; Zhang, X. Astaxanthin absorption modulated antioxidant enzyme activity and targeted specific metabolic pathways in rats. J. Sci. Food Agric. 2022, 102, 7003–7016. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Wang, W.; Liu, T.; Liu, Y.; Yu, L.; Yan, X.; Weng, W.; Lu, X.; Zhang, C. Astaxanthin attenuates alcoholic cardiomyopathy via inhibition of endoplasmic reticulum stress-mediated cardiac apoptosis. Toxicol. Appl. Pharmacol. 2021, 412, 115378. [Google Scholar] [CrossRef]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, J.; Zheng, G.; Zhao, M.; Hao, Z.; Lian, H.; Li, Y.; Wu, W.; Zhang, X.; Wang, J. Ochratoxin A induces cytotoxicity through ROS-mediated endoplasmic reticulum stress pathway in human gastric epithelium cells. Toxicology 2022, 479, 153309. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Friebel, A.; Brackhagen, L.; Hobloss, Z.; Myllys, M.; González, D.; Albrecht, W.; Mohammed, E.S.I.; Seddek, A.L.; Marchan, R.; et al. Hypoalbuminemia affects the spatio-temporal tissue distribution of ochratoxin A in liver and kidneys: Consequences for organ toxicity. Arch. Toxicol. 2022, 96, 2967–2981. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Li, H.X.; Yang, T.N.; Li, X.W.; Huang, Y.Q.; Zhu, S.Y.; Li, J.L. Di-(2-ethylhexyl) phthalate induced developmental abnormalities of the ovary in quail (Coturnix japonica) via disruption of the hypothalamic-pituitary-ovarian axis. Sci. Total Environ. 2020, 741, 140293. [Google Scholar] [CrossRef]
- Wu, Y.C.; Huang, H.H.; Wu, Y.J.; Manousakas, I.; Yang, C.C.; Kuo, S.M. Therapeutic and Protective Effects of Liposomal Encapsulation of Astaxanthin in Mice with Alcoholic Liver Fibrosis. Int. J. Mol. Sci. 2019, 20, 4057. [Google Scholar] [CrossRef] [PubMed]
- Humpage, A.R.; Fontaine, F.; Froscio, S.; Burcham, P.; Falconer, I.R. Cylindrospermopsin genotoxicity and cytotoxicity: Role of cytochrome P-450 and oxidative stress. J. Toxicol. Environ. Health Part A 2005, 68, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Huang, Y.; Lv, M.; Zhang, C.; Talukder, M.; Li, J.; Li, J. Cadmium induced Fak -mediated anoikis activation in kidney via nuclear receptors (AHR/CAR/PXR)-mediated xenobiotic detoxification pathway. J. Inorg. Biochem. 2022, 227, 111682. [Google Scholar] [CrossRef] [PubMed]
- Burkina, V.; Rasmussen, M.K.; Pilipenko, N.; Zamaratskaia, G. Comparison of xenobiotic-metabolising human, porcine, rodent, and piscine cytochrome P450. Toxicology 2017, 375, 10–27. [Google Scholar] [CrossRef]
- Knockaert, L.; Fromenty, B.; Robin, M.A. Mechanisms of mitochondrial targeting of cytochrome P450 2E1: Physiopathological role in liver injury and obesity. FEBS J. 2011, 278, 4252–4260. [Google Scholar] [CrossRef]
- Crespo, I.; García-Mediavilla, M.V.; Almar, M.; González, P.; Tuñón, M.J.; Sánchez-Campos, S.; González-Gallego, J. Differential effects of dietary flavonoids on reactive oxygen and nitrogen species generation and changes in antioxidant enzyme expression induced by proinflammatory cytokines in Chang Liver cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 1555–1569. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. NMCD 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Meki, A.R.; Hussein, A.A. Melatonin reduces oxidative stress induced by ochratoxin A in rat liver and kidney. Comparative biochemistry and physiology. Toxicol. Pharmacol. CBP 2001, 130, 305–313. [Google Scholar]
- Chen, J.T.; Kotani, K. Astaxanthin as a Potential Protector of Liver Function: A Review. J. Clin. Med. Res. 2016, 8, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bae, M.; Kim, B.; Park, Y.K.; Koo, S.I.; Lee, J.Y. Astaxanthin prevents and reverses the activation of mouse primary hepatic stellate cells. J. Nutr. Biochem. 2016, 29, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Darwish, W.S.; Ikenaka, Y.; Miki, W.; Ishizuka, M. Astaxanthin can alter CYP1A-dependent activities via two different mechanisms: Induction of protein expression and inhibition of NADPH P450 reductase dependent electron transfer. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Li, Q.; Zhang, T.; Wang, D.; Yang, L.; Xu, J.; Yanagita, T.; Xue, C.; Chang, Y.; Wang, Y. Effects of Astaxanthin and Docosahexaenoic-Acid-Acylated Astaxanthin on Alzheimer’s Disease in APP/PS1 Double-Transgenic Mice. J. Agric. Food Chem. 2018, 66, 4948–4957. [Google Scholar] [CrossRef] [PubMed]
- Pagliassotti, M.J. Endoplasmic reticulum stress in nonalcoholic fatty liver disease. Annu. Rev. Nutr. 2012, 32, 17–33. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Eletto, D.; Dersh, D.; Argon, Y. GRP94 in ER quality control and stress responses. Semin. Cell Dev. Biol. 2010, 21, 479–485. [Google Scholar] [CrossRef]
- Dong, L.; Tan, C.W.; Feng, P.J.; Liu, F.B.; Liu, D.X.; Zhou, J.J.; Chen, Y.; Yang, X.X.; Zhu, Y.H.; Zhu, Z.Q. Activation of TREM-1 induces endoplasmic reticulum stress through IRE-1α/XBP-1s pathway in murine macrophages. Mol. Immunol. 2021, 135, 294–303. [Google Scholar] [CrossRef]
- Rana, S.V.S. Endoplasmic Reticulum Stress Induced by Toxic Elements-a Review of Recent Developments. Biol. Trace Elem. Res. 2020, 196, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, H.M.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.D.; Miao, H.; Zhang, K.; Wolfson, A.; Pennathur, S.; Pipe, S.W.; Kaufman, R.J. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl. Acad. Sci. USA 2008, 105, 18525–18530. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.M.; Titus, E.A.; Cooper, A.A. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell 2004, 15, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Song, Y.S. Mitochondrial dynamics altered by oxidative stress in cancer. Free. Radic. Res. 2016, 50, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [PubMed]
- Wada, J.; Nakatsuka, A. Mitochondrial Dynamics and Mitochondrial Dysfunction in Diabetes. Acta Med. Okayama 2016, 70, 151–158. [Google Scholar]
- Patten, D.A.; McGuirk, S.; Anilkumar, U.; Antoun, G.; Gandhi, K.; Parmar, G.; Iqbal, M.A.; Wong, J.; Richardson, R.B.; St-Pierre, J.; et al. Altered mitochondrial fusion drives defensive glutathione synthesis in cells able to switch to glycolytic ATP production. Biochim. Biophys. Acta—Mol. Cell Res. 2021, 1868, 118854. [Google Scholar] [CrossRef]
- Shutt, T.; Geoffrion, M.; Milne, R.; McBride, H.M. The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep. 2012, 13, 909–915. [Google Scholar] [CrossRef]
- Coronado, M.; Fajardo, G.; Nguyen, K.; Zhao, M.; Kooiker, K.; Jung, G.; Hu, D.Q.; Reddy, S.; Sandoval, E.; Stotland, A.; et al. Physiological Mitochondrial Fragmentation Is a Normal Cardiac Adaptation to Increased Energy Demand. Circ. Res. 2018, 122, 282–295. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, K.F.; Chen, F.J.; Chen, Y.H.; Yang, X.; Cai, Z.H.; Jiang, Y.B.; Wang, X.B.; Zhang, G.P.; Wang, F.Y. Deoxynivalenol triggers porcine intestinal tight junction disorder: Insights from mitochondrial dynamics and mitophagy. Ecotoxicol. Environ. Saf. 2022, 248, 114291. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, W.; Wang, J.; Yang, X.; Zhang, Z.; Zhao, Z. T-2 Toxin-Induced Oxidative Stress Leads to Imbalance of Mitochondrial Fission and Fusion to Activate Cellular Apoptosis in the Human Liver 7702 Cell Line. Toxins 2020, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, T.; Deuster, P. Astaxanthin Protects Against Heat-induced Mitochondrial Alterations in Mouse Hypothalamus. Neuroscience 2021, 476, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Tolba, S.A.; Magnuson, A.D.; Sun, T.; Lei, X.G. Dietary supplemental microalgal astaxanthin modulates molecular profiles of stress, inflammation, and lipid metabolism in broiler chickens and laying hens under high ambient temperatures. Poult. Sci. 2020, 99, 4853–4860. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Zhang, S.; Yang, J.; Qin, C.; Jin, B.; Liang, Z.; Yang, S.; Li, L.; Long, M. Protective Effects of Astaxanthin on Ochratoxin A-Induced Liver Injury: Effects of Endoplasmic Reticulum Stress and Mitochondrial Fission–Fusion Balance. Toxins 2024, 16, 68. https://doi.org/10.3390/toxins16020068
Zou Y, Zhang S, Yang J, Qin C, Jin B, Liang Z, Yang S, Li L, Long M. Protective Effects of Astaxanthin on Ochratoxin A-Induced Liver Injury: Effects of Endoplasmic Reticulum Stress and Mitochondrial Fission–Fusion Balance. Toxins. 2024; 16(2):68. https://doi.org/10.3390/toxins16020068
Chicago/Turabian StyleZou, Yiting, Shiyi Zhang, Jian Yang, Chen Qin, Bo Jin, Zhenyu Liang, Shuhua Yang, Lin Li, and Miao Long. 2024. "Protective Effects of Astaxanthin on Ochratoxin A-Induced Liver Injury: Effects of Endoplasmic Reticulum Stress and Mitochondrial Fission–Fusion Balance" Toxins 16, no. 2: 68. https://doi.org/10.3390/toxins16020068
APA StyleZou, Y., Zhang, S., Yang, J., Qin, C., Jin, B., Liang, Z., Yang, S., Li, L., & Long, M. (2024). Protective Effects of Astaxanthin on Ochratoxin A-Induced Liver Injury: Effects of Endoplasmic Reticulum Stress and Mitochondrial Fission–Fusion Balance. Toxins, 16(2), 68. https://doi.org/10.3390/toxins16020068