Porphyromonas gingivalis Gingipains Trigger a Proinflammatory Response in Human Monocyte-derived Macrophages Through the p38α Mitogen-activated Protein Kinase Signal Transduction Pathway
Abstract
:1. Introduction
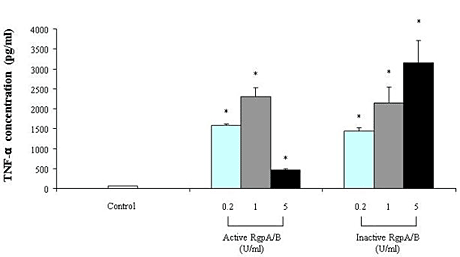
2. Results




| Protein | Signal (% Change) | ||
|---|---|---|---|
| Full name and abbreviation | Epitope | Unstimulated vs. RgpA/B | Unstimulated vs. Kgp |
| Extracellular regulated protein-serine kinase 1/2 (p42, p44 MAP kinase); ERK1/2 | T202/Y204 | +25 | +32 |
| Jun N-terminus protein-serine kinase (stress-activated protein kinase 2); JNK2 | T183/Y185 | +5 | 0 |
| Mitogen-activated protein-serine kinase p38 alpha; p38α MAPK | T180/Y182 | +343 | +227 |
| Gingipain preparation (1 unit/mL) | % inhibition in the presence of SB203580 | |
|---|---|---|
| TNF-α | IL-8 | |
| RgpA/B | 87% | 92% |
| Kgp | 79% | 69% |
3. Discussion
4. Experimental Section
4.1. Bacterial strains and growth conditions
4.2. Proteinase purification
4.3. Preparation and stimulation of monocyte-derived macrophages
4.4. Macrophage viability
4.5. Determination of TNF- and IL-8 secretion
4.6. Analysis of phosphorylated macrophage intracellular kinases
4.7. Effect of a p38 MAPK inhibitor on macrophage activation by gingipains
5. Conclusions
Acknowledgements
References
- Schenkein, H.A. Host responses in maintaining periodontal health and determining periodontal disease. Periodontol. 2000 2006, 40, 77–93. [Google Scholar] [CrossRef]
- Slots, J.; Ting, M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol. 2000 1999, 20, 82–121. [Google Scholar] [CrossRef]
- Cutler, C.W.; Calmer, J.R.; Genco, C.A. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis. Trends Microbiol. 1995, 3, 45–51. [Google Scholar] [CrossRef]
- Grenier, D.; Mayrand, D. Periodontitis as an ecological imbalance. In Oral Bacterial Ecology: The Molecular Basis; Kuramitsu, H.K., Ellen, R., Eds.; Horizon Scientific Press: Wymondham,UK, 2000; pp. 275–311. [Google Scholar]
- Kadowaki, T.; Nakayama, K.; Okamoto, K.; Abe, N.; Baba, A.; Shi, Y.; Ratnayake, D.B.; Yamamoto, K. Porphyromonas gingivalis proteinases as virulence determinants in progression of periodontal diseases. J. Biochem. 2000, 128, 153–159. [Google Scholar]
- Potempa, J.; Sroka, A.; Imamura, T.; Travis, J. Gingipains, the major cysteine proteinase and virulence factors of Porphyromonas gingivalis: Structure, function and assembly of multidomain protein complexes. Curr. Protein Pept. Sci. 2003, 4, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Andrian, E.; Grenier, D.; Rouabhia, M. In vitro models of tissue penetration and destruction by Porphyromonas gingivalis. Infect. Immun. 2004, 72, 4689–4698. [Google Scholar] [CrossRef]
- Houle, M.A.; Grenier, D.; Plamondon, P.; Nakayama, K. The collagenase activity of Porphyromonas gingivalis is due to Arg-gingipain. FEMS Microbiol. Lett. 2003, 221, 181–185. [Google Scholar] [CrossRef]
- Grayson, R.; Douglas, C.W.; Heath, J.; Rawlinson, A.; Evans, G.S. Activation of human matrix metalloproteinase by gingival crevicular fluid and Porphyromonas gingivalis. J. Clin. Periodontol. 2003, 30, 542–550. [Google Scholar] [CrossRef]
- Grenier, D.; Mayrand, D. Inactivation of tissue inhibitor of metalloproteinase-1 (TIMP-1) by Porphyromonas gingivalis. FEMS Microbiol. Lett. 2001, 203, 161–164. [Google Scholar] [CrossRef]
- Zappa, U.; Reinking-Zappa, M.; Graf, H.; Espeland, M. Cell populations and episodic periodontal attachment loss in humans. J. Clin. Periodontol. 1991, 18, 508–515. [Google Scholar] [CrossRef]
- Bodet, C.; Chandad, F.; Grenier, D. Modulation of cytokine production by Porphyromonas gingivalis in a macrophage and epithelial cell co-culture model. Microbes Infect. 2005, 7, 448–456. [Google Scholar] [CrossRef]
- Sugano, N.; Ikeda, K.; Oshikawa, M.; Sawamoto, Y.; Tanaka, H.; Ito, K. Differential cytokine induction by two types of Porphyromonas gingivalis. Oral Microbiol. Immunol. 2004, 19, 121–123. [Google Scholar] [CrossRef]
- O’Brien-Simpson, N.M.; Veith, P.D.; Dashper, S.G.; Reynolds, E.C. Antigens of bacteria associated with periodontitis. Periodontol. 2000 2004, 35, 101–134. [Google Scholar] [CrossRef]
- Pathirana, R.D.; O’Brien-Simpson, N.M.; Brammar, G.C.; Slakeski, N.; Reynolds, E.C. Kgp and RgpB, but not RgpA, are important for Porphyromonas gingivalis virulence in the murine periodontitis model. Infect. Immun. 2007, 75, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Hirofuji, T.; Motooka, N.; Anan, H.; Miura, M.; Ishihara, Y.; Maeda, K. Antibody responses to Porphyromonas gingivalis infection in a murine abscess model - involvement of gingipains and responses to re-infection. J. Periodontal Res. 2003, 38, 551–556. [Google Scholar] [CrossRef]
- Imamura, T.; Pike, R.N.; Potempa, J.; Travis, J. Pathogenesis of periodontitis: a major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of the kallikrein/kinin pathway. J. Clin. Invest. 1994, 94, 361–367. [Google Scholar] [CrossRef]
- Imamura, T.; Potempa, J.; Tanase, S.; Travis, J. Activation of blood coagulation factor X by arginine-specific cysteine proteinases (gingipain-Rs) from Porphyromonas gingivalis. J. Biol. Chem. 1997, 272, 16062–16067. [Google Scholar]
- Senden, N.H.; Jeunhomme, T.M.; Heemskerk, J.W.; Wagenvoord, R.; van’t Veer, C.; Hemker, H.C.; Buurman, W.A. Factor Xa induces cytokine production and expression of adhesion molecules by human umbilical vein endothelial cells. J. Immunol. 1998, 161, 4318–4324. [Google Scholar]
- O’Brien-Simpson, N.M.; Pathirana, R.D.; Walker, G.D.; Reynolds, E.C. Porphyromonas gingivalis RgpA-Kgp proteinase-adhesin complexes penetrate gingival tissue and induce proinflammatory cytokines or apoptosis in a concentration-dependent manner. Infect. Immun. 2009, 77, 1246–1261. [Google Scholar]
- Uehara, A.; Imamura, T.; Potempa, J.; Travis, J.; Takada, H. Gingipains of Porphyromonas gingivalis synergistically induce the production of proinflammatory cytokines through protease-activated receptors with Toll-like receptor and NOD1/2 ligands in human monocytic cells. Cell Microbiol. 2008, 10, 1181–1189. [Google Scholar] [CrossRef]
- Fitzpatrick, R.E.; Aprico, A.; Wijeyewickrema, L.C.; Pagel, C.N.; Wong, D.M.; Potempa, J.; Mackie, E.J.; Pike, R.N. High molecular weight gingipains from Porphyromonas gingivalis induce cytokine responses from human macrophage-like cells via a nonproteolytic mechanism. J. Innate Immun. 2009, 1, 109–117. [Google Scholar] [CrossRef]
- Graves, D.T.; Cochran, D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef]
- Silva, T.A.; Garlet, G.P.; Fukada, S.Y.; Silva, J.S.; Cunha, F.Q. Chemokines in oral inflammatory diseases: Apical periodontitis and periodontal disease. J. Dent. Res. 2007, 86, 306–319. [Google Scholar] [CrossRef]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef]
- Jin, L.J.; Leung, W.K.; Corbet, E.F.; Soder, B. Relationship of changes in interleukin-8 levels and granulocyte elastase activity in gingival crevicular fluid to subgingival periodontopathogens following non-surgical periodontal therapy in subjects with chronic periodontitis. J. Clin. Periodontol. 2002, 29, 604–614. [Google Scholar] [CrossRef]
- Stashenko, P.; Jandinski, J.J.; Fujiyoshi, P.; Rynar, J.; Socransky, S.S. Tissue levels of bone resorptive cytokines in periodontal disease. J. Periodontol. 1991, 62, 504–509. [Google Scholar] [CrossRef]
- Calkins, C.C.; Platt, K.; Potempa, J.; Travis, J. Inactivation of tumor necrosis factor-alpha by proteinases (gingipains) from the periodontal pathogen, Porphyromonas gingivalis. Implications of immune evasion. J. Biol. Chem. 1998, 273, 6611–6614. [Google Scholar]
- Mikolajczyk-Pawlinska, J.; Travis, J.; Potempa, J. Modulation of interleukin-8 activity by gingipains from Porphyromonas gingivalis: Implications for pathogenicity of periodontal disease. FEBS Lett. 1998, 440, 282–286. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar]
- Patil, C.S.; Kirkwood, K.L. p38 MAPK signalling in oral-related diseases. J. Dent. Res. 2007, 86, 812–825. [Google Scholar] [CrossRef]
- Nakayama, K.; Kadowaki, T.; Okamoto, K.; Yamamoto, Y. Construction and characterization of arginine-specific cysteine proteinase (arg-gingipain)-deficient mutants of Porphyromonas gingivalis. J. Biol. Chem. 1995, 270, 23619–23626. [Google Scholar]
- Shi, Y.; Ratnayake, D.B.; Okamoto, K.; Abe, N.; Yamamoto, K.; Nakayama, K. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis: Construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J. Biol. Chem. 1999, 274, 17955–17960. [Google Scholar] [PubMed]
- Fujimura, S.; Hirai, K.; Shibata, Y.; Nakayama, K.; Nakamura, T. Comparative properties of envelope-associated arginine-gingipains and lysine-gingipain of Porphyromonas gingivalis. FEMS Microbiol. Lett. 1998, 163, 173–179. [Google Scholar] [CrossRef]
- Pike, R.; McGraw, W.; Potempa, J.; Travis, J. 1994. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J. Biol. Chem. 1994, 269, 406–411. [Google Scholar] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Grenier, D.; Tanabe, S.-i. Porphyromonas gingivalis Gingipains Trigger a Proinflammatory Response in Human Monocyte-derived Macrophages Through the p38α Mitogen-activated Protein Kinase Signal Transduction Pathway. Toxins 2010, 2, 341-352. https://doi.org/10.3390/toxins2030341
Grenier D, Tanabe S-i. Porphyromonas gingivalis Gingipains Trigger a Proinflammatory Response in Human Monocyte-derived Macrophages Through the p38α Mitogen-activated Protein Kinase Signal Transduction Pathway. Toxins. 2010; 2(3):341-352. https://doi.org/10.3390/toxins2030341
Chicago/Turabian StyleGrenier, Daniel, and Shin-ichi Tanabe. 2010. "Porphyromonas gingivalis Gingipains Trigger a Proinflammatory Response in Human Monocyte-derived Macrophages Through the p38α Mitogen-activated Protein Kinase Signal Transduction Pathway" Toxins 2, no. 3: 341-352. https://doi.org/10.3390/toxins2030341
APA StyleGrenier, D., & Tanabe, S. -i. (2010). Porphyromonas gingivalis Gingipains Trigger a Proinflammatory Response in Human Monocyte-derived Macrophages Through the p38α Mitogen-activated Protein Kinase Signal Transduction Pathway. Toxins, 2(3), 341-352. https://doi.org/10.3390/toxins2030341