Degradation of Aflatoxin B1 during the Fermentation of Alcoholic Beverages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Validation
| RSD (%) | Linearity (r) | Recovery (%) | |
|---|---|---|---|
| Cool wort | 5.5 | 1.00 | 87 |
| Beer | 1.7 | 1.00 | 88 |
| Yeast crop | 4.3 | 1.00 | 43 |
| Must | 5.7 | 1.00 | 91 |
| Wine | 4.4 | 1.00 | 96 |
| Wine lees | 4.5 | 1.00 | 71 |
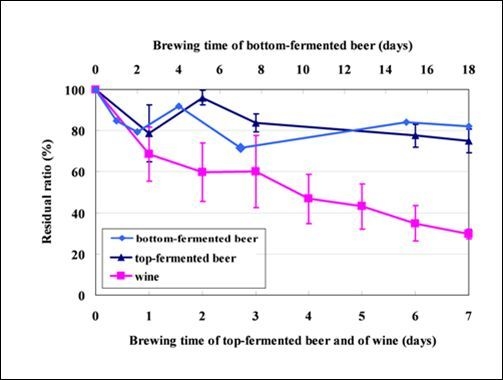
2.2. Fate of Mycotoxins during Beer Brewing

2.3. Fate of Aflatoxins during Vinification
2.4. Identification of Degradation Compounds



2.5. Aflatoxin B2a during Beer Fermentation
3. Experimental Section
3.1. Materials
3.2. Apparatus
3.3. Analytical Method
3.3.1. High-Sensitivity Tandem Mass Spectrometry
3.3.2. High-Resolution Tandem Mass Spectrometry
3.4. Laboratory-Scale Fermentation
3.4.1. Laboratory-Scale Beer Fermentation
3.4.2. Laboratory-Scale Wine Vinification
3.5. Sample Preparation
3.5.1. Sample Preparation for Beer
3.5.2. Sample Preparation for Wine
3.6. Synthesis of Aflatoxin B2a
3.7. Method Validation
3.8. Analysis
4. Conclusions
Conflict of Interest
References
- Nagatomi, Y.; Inoue, T.; Uyama, A.; Mochizuki, N. The fate of mycotoxin during the distillation process of barley shochu, a distilled alcoholic beverage. Biosci. Biotechnol. Biochem. 2012, 76, 202–204. [Google Scholar] [CrossRef]
- Mizutani, K.; Nagatomi, Y.; Mochizuki, N. Metabolism of zearalenone in the course of beer fermentation. Toxins 2011, 3, 134–141. [Google Scholar]
- Inoue, T.; Nagatomi, Y.; Uyama, A.; Mochizuki, N. Fate of mycotoxins during beer brewing and fermentation. Biosci. Biotechnol. Biochem. 2013, in press. [Google Scholar]
- World Health Organization International Agency for Research on Cancer IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 82. 2002. Available online: http://monographs.iarc.fr/ENG/Monographs/vol82/mono82.pdf (accessed on 18 March 2013).
- Forrester, L.M.; Neal, G.E.; Judah, D.J.; Glancey, M.J.; Wolf, C.R. Evidence for involvement of multiple forms of cytochrome P-450 in aflatoxin B1 metabolism in human liver. Proc. Natl. Acad. Sci. USA 1990, 87, 8306–8310. [Google Scholar]
- Essigmann, J.M.; Croy, R.G.; Nadzan, A.M.; Busby, W.F., Jr.; Reinhold, V.N.; Buchi, G.; Wogan, G.N. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc. Natl. Acad. Sci. USA 1977, 74, 1870–1874. [Google Scholar]
- Martin, C.N.; Garner, R.C. Aflatoxin B-oxide generated by chemical or enzymic oxidation of aflatoxin B1 causes guanine substitution in nucleic acids. Nature 1977, 267, 863–865. [Google Scholar] [CrossRef]
- Mably, M.; Mankotia, M.; Cavlovic, P.; Tam, J.; Wong, L.; Pantazopoulos, P.; Calway, P.; Scott, P.M. Survey of aflatoxins in beer sold in Canada. Food Addit. Contam. 2005, 22, 1252–1257. [Google Scholar]
- Nakajima, M.; Tsubouchi, H.; Miyabe, M. A survey of ochratoxin A and aflatoxins in domestic and imported beers in Japan by immunoaffinity and liquid chromatography. J. AOAC Int. 1999, 82, 897–902. [Google Scholar]
- Reddy, K.R.N.; Salleh, B. A preliminary study on the occurrence of Aspergillus spp. and aflatoxin B1 in imported wheat and barley in Penang, Malaysia. Mycotoxin Res 2010, 26, 267–271. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, E.K.; Shon, D.H.; Kim, Y.B. Natural co-occurrence of aflatoxin B1, fumonisin B1 and ochratoxin A in barley and corn foods from Korea. Food Addit. Contam. 2002, 19, 1073–1080. [Google Scholar] [CrossRef]
- Scudamore, K.A.; Patel, S. Survey for aflatoxins, ochratoxin A, zearalenone and fumonisins in maize imported into United Kingdom. Food Addit. Contam. 2000, 17, 407–416. [Google Scholar] [CrossRef]
- Lewis, L.; Onsongo, M.; Njapau, H.; Shurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A.; et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767. [Google Scholar]
- Khoury, A.E.; Rizk, T.; Lteif, R.; Azouri, H.; Delia, M.L.; Lebrishi, A. Occurrence of ochratoxin A- and aflatoxin B1-producing fungi in Lebanese grapes and ochratoxin A content in musts and finished wines during 2004. J. Agric. Food Chem. 2006, 54, 8977–8982. [Google Scholar] [CrossRef]
- Khoury, A.E.; Rizk, T.; Lteif, R.; Azouri, H.; Delia, M.L.; Lebrishi, A. fungal contamination and aflatoxin B1 and ochratoxin A in Lebanese wine-grapes and musts. Food Chem. Toxicol. 2008, 46, 2244–2250. [Google Scholar] [CrossRef]
- Chris, B.; David, Q. Brewing Yeast and Fermentation, 1st ed; Wiley-Blackwell: Oxford, UK, 2006; pp. 168–171, 283, 424–426. [Google Scholar]
- Takahashi, D.M. High pressure liquid chromatographic determination of aflatoxins in wines and other liquid products. J. Assoc. Off. Anal. Chem. 1977, 60, 799–804. [Google Scholar]
- Ciegler, A.; Peterson, R.E. Aflatoxin detoxification: Hydroxydihydro-aflatoxin B1. Appl. Microbiol. 1968, 16, 665–666. [Google Scholar]
- Lillehoj, E.B.; Ciegler, A. Biological activity of aflatoxin B2a. Appl. Microbiol. 1969, 17, 516–519. [Google Scholar]
- Tamura, M.; Takahashi, A.; Uyama, A.; Mochizuki, N. A method for multiple mycotoxin analysis in wines by solid phase extraction and multifunctional cartridge purification, and ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. Toxins 2012, 4, 476–486. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Inoue, T.; Nagatomi, Y.; Uyama, A.; Mochizuki, N. Degradation of Aflatoxin B1 during the Fermentation of Alcoholic Beverages. Toxins 2013, 5, 1219-1229. https://doi.org/10.3390/toxins5071219
Inoue T, Nagatomi Y, Uyama A, Mochizuki N. Degradation of Aflatoxin B1 during the Fermentation of Alcoholic Beverages. Toxins. 2013; 5(7):1219-1229. https://doi.org/10.3390/toxins5071219
Chicago/Turabian StyleInoue, Tomonori, Yasushi Nagatomi, Atsuo Uyama, and Naoki Mochizuki. 2013. "Degradation of Aflatoxin B1 during the Fermentation of Alcoholic Beverages" Toxins 5, no. 7: 1219-1229. https://doi.org/10.3390/toxins5071219
APA StyleInoue, T., Nagatomi, Y., Uyama, A., & Mochizuki, N. (2013). Degradation of Aflatoxin B1 during the Fermentation of Alcoholic Beverages. Toxins, 5(7), 1219-1229. https://doi.org/10.3390/toxins5071219