Detection of Dinophysistoxin-1 in Clonal Culture of Marine Dinoflagellate Prorocentrum foraminosum (Faust M.A., 1993) from the Sea of Japan
Abstract
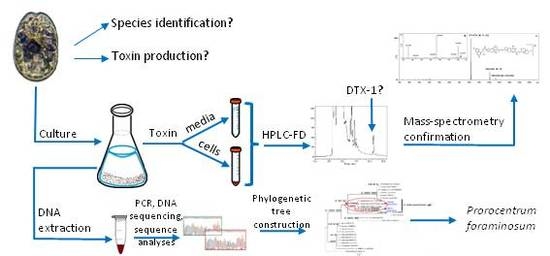
:1. Introduction
2. Results and Discussion





| Species | Toxin | “Location” | Concentration | Reference |
|---|---|---|---|---|
| P. lima | OA | field cells | 4–6.4 pg/cell | [23] |
| algal sample | 21 ng/mg | [24] | ||
| culture cells | 1.3–26 pg/cell | [8,25,26] | ||
| culture media | 25 µg/L | [27] | ||
| DTX-1 | culture cells | 4–14.3 pg/cell | [25,26] | |
| culture media | 25 µg/L | [27] | ||
| P. belizeanum | OA | culture cells | 12.45 pg/cell | [28] |
| P. hoffmannianum | OA | culture cells and media | 500–800 µg/L | [29] |
| P. concavum | OA | culture cells | 10–15 pg/cell | [8] |
| P. rhathymum | OA | culture media | 0.153 µg/L | [14,15] |
| P. foraminosum | DTX-1 | culture cells | 8.4 ± 2.5 pg/cell | present study |
| culture media | 27.9 ± 14.7 µg/L |
3. Experimental Section
3.1. Reagents and Standards
3.2. Microalgae Collection
3.3. Isolation, Culturing and Cell Number Estimation
3.4. Molecular Identification of Species
3.5. Toxin Extraction
3.5.1. Cells
3.5.2. Media
3.5.3. General
3.6. Derivatization and Purification
3.7. High Performance Liquid-Chromatography with Fluorescent Detection (HPLC-FLD)
3.8. Toxin Concentration Estimation
3.9. High Performance Liquid Chromatography-High Resolution Mass-Spectrometry (HPLC-HRMS)
4. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Vershinin, A.; Moruchkov, A.; Morton, S.; Leighfield, T.; Quilliam, M.; Ramsdell, J. Phytoplankton composition of the Kandalaksha Gulf, Russian White Sea: Dinophysis and lipophilic toxins in the blue mussel (Mytilus edulis). Harmful Algae 2006, 5, 558–564. [Google Scholar]
- Morton, S.; Vershinin, A.; Laurinda, L.S.; Leighfield, T.A.; Pankov, S.; Quilliam, M.A. Seasonality of Dinophysis spp. and Prorocentrum lima in Black Sea phytoplankton and associated shellfish toxicity. Harmful Algae 2009, 8, 629–636. [Google Scholar] [CrossRef]
- Orlova, T.; Morozova, T.; Kameneva, P.; Shevchenko, O. Harmful algal blooms on the Russian east coast and their possible economic impacts. PICES Sci. Rep. 2014, 47, 41–58. [Google Scholar]
- Kameneva, P.A.; Imbs, A.B.; Orlova, T.Y. Distribution of DTX-3 in edible and non-edible parts of Crenomytilus grayanus from the Sea of Japan. Toxicon 2015, 93, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Reguera, B.; Riobo, P.; Rodriguez, F.; Diaz, P.A.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef] [PubMed]
- Hoppenrath, M.; Chomerat, N.; Horiguchi, T.; Schweikert, M.; Nagahama, Y.; Murray, S. Taxonomy and phylogeny of the benthic Prorocentrum species (Dinophyceae)—A proposal and review. Harmful Algae 2013, 27, 1–28. [Google Scholar] [CrossRef]
- Ten-Hage, L.; Robillot, C.; Turquet, J.; Le Gall, F.; Le Caer, J.P.; Bultel, V.; Molgo, J. Effects of toxic extracts and purified borbotoxins from Prorocentrum borbonicum (Dinophyceae) on vertebrate neuromuscular junctions. Toxicon 2002, 40, 137–148. [Google Scholar] [CrossRef]
- Hu, T.; Marr, J.; De Freitas, A.S.W.; Quilliam, M.A.; Wright, J.L.C.; Pleasance, S. New diolesters (of okadaic acid) isolated from cultures of the dinoflagellates P. lima and P. concavum. J. Nat. Prod. 1992, 55, 1631–1637. [Google Scholar] [CrossRef]
- Hu, T.; De Freitas, A.S.W.; Doyle, J.; Jackson, D.; Marr, J.; Nixon, E.; Pleasance, S.; Quilliam, M.A.; Walter, J.A.; Wright, J.L.C. New DSP toxin derivatives isolated from toxic mussels and the dinoflagellates. In Prorocentrum lima and Prorocentrum concavum. Toxic Phytoplankton Blooms in the Sea; Elsevier: Amsterdam, Netherland, 1993; pp. 507–512. [Google Scholar]
- Yasumoto, T.; Seino, N.; Murakami, Y.; Murata, M. Toxins produced by benthic dinoflagellates. Biol. Bull. 1987, 172, 128–131. [Google Scholar] [CrossRef]
- Morton, S.L.; Leighfield, T.A.; Haynes, B.L.; Petitpain, D.L.; Busman, M.A.; Moeller, P.D.R.; Bean, L.; McGowan, J.; Hurst, J.W.; Van Dolah, J.R. Evidence of diarrhetic shellfish poisoning along the coast of Maine. J. Shell. Res. 1999, 18, 681–686. [Google Scholar]
- Jackson, A.E.; Marr, J.C.; McLachlan, J.L. The production of diarrhetic shellfish toxins by an isolate of Prorocentrum lima from Nova Scotia, Canada. In Toxic Phytoplankton Blooms in the Sea; Elsevier: New York, NY, USA, 1993; pp. 513–518. [Google Scholar]
- Lawrence, J.E.; Bauder, A.G.; Quilliam, M.A.; Cembella, A.D. Prorocentrum lima: A putative link to diarrhetic shellfish poisoning in Nova Scotia, Canada. In Harmful Microalgae; Xunta de Galicia and UNESCO: Santiago de Compostela, Spain, 1998; pp. 78–79. [Google Scholar]
- An, T.; Winshell, J.; Scorzetti, G.; Fell, J.W.; Rein, K.S. Identification of okadaic acid production in the marine dinoflagellate Prorocentrum rhathymum from Florida Bay. Toxicon 2010, 55, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, A.; De La Iglesia, P.; Campas, M.; Elandaloussi, L.; Fernandez, M.; Mohammad-Noor, N.; Andree, K.; Diogene, J. Evidence of okadaic acid production in a cultured strain of the marine dinoflagellate Prorocentrum rhathymum from Malaysia. Toxicon 2010, 55, 633–637. [Google Scholar]
- Faust, M.A. Three new benthic species of Prorocentrum (Dinophyceae) from Twin Cays, Belize: P. maculosum sp. nov., P. foraminosum sp. nov. and P. formosum sp. nov. Phycologia 1993, 32, 410–418. [Google Scholar] [CrossRef]
- Windust, A.J.; Quilliam, M.A.; Wright, J.L.C.; McLachlan, J.L. Comparitive toxicity of the diarrhetic shellfish poisons, okadaic acid, okadaic acid diol ester and dinophysistoxin-4, to the diatom Thalassiosira weissflogii. Toxicon 1997, 35, 1591–1603. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Hawkes, A.D.; Jensen, D.J.; Cooney, J.M.; Larsen, K.; Petersen, D.; Rise, F.; Beuzenberg, V.; MacKenzie, A.L. Isolation and identification of a cis-C8-diol-ester of okadaic acid from Dinophysis acuta in New Zealand. Toxicon 2006, 2, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Marr, J.C.; McDowell, L.M.; Quilliam, M.A. Investigation of derivatization reagents for the analysis of diarrhetic shellfish poisoning toxins by liquid chromatography with fluorescence detection. Nat. Toxins 1994, 2, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Torgersen, T.; Wilkins, A.L.; Rundberget, T.; Miles, C.O. Characterization of fatty acid esters of okadaic acid and related toxins in blue mussels (Mytilus edulis) from Norway. Rapid Commun. Mass Spectrom. 2008, 22, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Aligizaki, K.; Nikolaidis, G.; Katikou, P.; Baxevanis, A.D.; Abatzopoulos, T.J. Potentially toxic epiphytic Prorocentrum (Dinophyceae) species in Greek coastal waters. Harmful Algae 2009, 8, 299–311. [Google Scholar] [CrossRef]
- David, H.; Laza-Martınez, A.; Garcıa-Etxebarria, K.; Riobo, P.; Orive, E. Characterization of Prorocentrum elegans and Prorocentrum levis (Dinophyceae) from the south-eastern bay of Biscay by morphology and molecular phylogeny. J. Phycol. 2014, 50, 718–726. [Google Scholar] [CrossRef]
- Rhodes, L.; Syhre, M. Okadaic acid production by a New Zealand Prorocentrum lima isolate. New Zeal. J. Mar. Fresh. 1995, 29, 367–370. [Google Scholar] [CrossRef]
- Ten-Hage, L.; Turquet, J.; Quod, J.-P.; Puiseux-Dao, S.; Coute, A. Prorocentrum borbonicum sp. nov. (Dinophyceae), a new toxic benthic dinoflagellate from the southwestern Indian Ocean. Phycologia 2000, 39, 296–301. [Google Scholar] [CrossRef]
- Lee, J.; Igarashi, T.; Fraga, S.; Dahl, E.; Hovgaard, P.; Yasumoto, T. Determination of diarrhetic shellfish toxins in various dinoflagellate species. J. Appl. Phycol. 1989, 1, 147–152. [Google Scholar] [CrossRef]
- Morton, S.L.; Tindall, D.R. Morphological and biochemical variability of the toxic dinoflagellate Prorocentrum lima isolated from three locations at Heron Island, Australia. J. Phycol. 1995, 31, 914–921. [Google Scholar] [CrossRef]
- Marr, J.C.; Jackson, A.E.; McLachlan, J.L. Occurrence of Prorocentrum lima, a DSP toxin-producing species from the Atlantic coast of Canada. J. Appl. Phycol. 1992, 4, 17–24. [Google Scholar] [CrossRef]
- Morton, S.L.; Moeller, P.D.R.; Young, K.A.; Lanoue, B. Okadaic acid production from the marine dinoflagellate Prorocentrum belizeanum Faust isolated from the Belizean coral reef ecosystem. Toxicon 1998, 36, 201–206. [Google Scholar] [CrossRef]
- Morton, S.L.; Bomber, J.W. Maximizing okadaic acid content from Prorocentrum hoffmannianum Faust. J. Appl. Phycol. 1994, 6, 41–44. [Google Scholar] [CrossRef]
- Selina, M.S.; Morozova, T.V.; Vyshkvartsev, D.I.; Orlova, T.Y. Seasonal dynamics and spatial distribution of epiphytic dinoflagellates in Peter the Great Bay (Sea of Japan) with special emphasis on Ostreopsis species. Harmful Algae 2013, 32, 1–10. [Google Scholar] [CrossRef]
- Montero-Pau, J.; Gomez, A.; Munoz, J. Application of an inexpensive and high-throughput genomic DNA extraction method for the molecular ecology of zooplanktonic diapausing eggs. Limnol. Oceanogr.-Meth. 2008, 6, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Efimova, K.V.; Orlova, T.Y.; Brykov, V.A. Phylogenetic characterization of cryptic species of the marine dinoflagellate, Ostreopsis sp. Shmidt, 1902, from Russian coastal waters, the Sea of Japan. JBES 2014, 5, 317–332. [Google Scholar]
- Chinain, M.; Faust, M.A.; Paullac, S. Morphology and molecular analyses of three species of Gambierdiscus (Dinophyceae): G. pacificus, sp. nov., G. australes, sp. nov., and G. polynesiensis, sp. nov. J. Phycol. 1999, 35, 1282–1296. [Google Scholar] [CrossRef]
- Imbs, A.B.; Vologodskaya, A.V.; Nevshupova, N.V.; Khotimchenko, S.V.; Titlyanov, E.A. Response of prostaglandin content in the red alga Gracilaria verrucosa to season and solar irradiance. Phytochemistry 2001, 58, 1067–1072. [Google Scholar] [CrossRef]
- McNaab, P. Chemistry, metabolism and chemical analysis of okadaic acid group toxins. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection, 2nd ed.; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 209–228. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kameneva, P.A.; Efimova, K.V.; Rybin, V.G.; Orlova, T.Y. Detection of Dinophysistoxin-1 in Clonal Culture of Marine Dinoflagellate Prorocentrum foraminosum (Faust M.A., 1993) from the Sea of Japan. Toxins 2015, 7, 3947-3959. https://doi.org/10.3390/toxins7103947
Kameneva PA, Efimova KV, Rybin VG, Orlova TY. Detection of Dinophysistoxin-1 in Clonal Culture of Marine Dinoflagellate Prorocentrum foraminosum (Faust M.A., 1993) from the Sea of Japan. Toxins. 2015; 7(10):3947-3959. https://doi.org/10.3390/toxins7103947
Chicago/Turabian StyleKameneva, Polina A., Kseniya V. Efimova, Viacheslav G. Rybin, and Tatiana Y. Orlova. 2015. "Detection of Dinophysistoxin-1 in Clonal Culture of Marine Dinoflagellate Prorocentrum foraminosum (Faust M.A., 1993) from the Sea of Japan" Toxins 7, no. 10: 3947-3959. https://doi.org/10.3390/toxins7103947
APA StyleKameneva, P. A., Efimova, K. V., Rybin, V. G., & Orlova, T. Y. (2015). Detection of Dinophysistoxin-1 in Clonal Culture of Marine Dinoflagellate Prorocentrum foraminosum (Faust M.A., 1993) from the Sea of Japan. Toxins, 7(10), 3947-3959. https://doi.org/10.3390/toxins7103947