Study of Adsorption and Flocculation Properties of Natural Clays to Remove Prorocentrum lima
Abstract
:1. Introduction
2. Results and Discussion




| Mean Cells ± SEM (n = 3) | |||||||
|---|---|---|---|---|---|---|---|
| Column Content | Time (Minutes) | ||||||
| 0 | 5 | 15 | 30 | 60 | 120 | 180 | |
| P. lima | 631 ± 7.1 | 447 ± 20.9 | 267 ± 26.5 | 149 ± 32.5 | 91 ± 30.8 | 56 ± 18 | 47 ± 14.8 |
| P. lima + bentonite > 45 µm | 557 ± 29.4 | 297 ± 4.9 | 156 ± 42.5 * | 74 ± 26.4 * | 25 ± 6.6 * | 16 ± 3.2 | 11 ± 2.3 |
| P. lima + Lendo clay | 521 ± 11.7 | 287 ± 22.6 | 81 ± 12 | 15 ± 8.1 | 3 ± 2 | 1 ± 0.3 | 0 |
| P. lima + Grove clay | 536 ± 11.9 | 215 ± 44.2 | 61 ± 10.9 | 14 ± 8.8 | 3 ± 2.3 | 0 | 0 |
| P. lima + bentonite | 557 ± 18.6 | 221 ± 40.6 | 49 ± 23.8 | 9 ± 2.4 | 2 ± 0.3 | 0 | 0 |
| P. lima + kaolinite | 441 ± 27.4 | 209 ± 31.3 | 48 ± 6.5 | 14 ± 3.8 | 2 ± 1.5 | 0 | 0 |
| P. lima + bentonite Na | 506 ± 13.8 | 141 ± 35.7 | 67 ± 20.3 | 9 ± 1.2 | 1 ± 0.3 | 0 | 0 |


| Column Content | Time (Minutes) | Clay Size | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 15 | 30 | 60 | 120 | 180 | ||
| P. lima + bentonite > 45 µm | 11.8 | 33.6 | 41.6 | 50.1 | 72.1 | 70.7 | 76 | >45 µm |
| P. lima + lendo clay | 17.4 | 35.8 | 69.5 | 89.7 | 96.7 | 98.8 | 100 | 57% < 2µm |
| P. lima + grove clay | 15 | 51.9 | 77.3 | 90.4 | 96.3 | 100 | 100 | 69% < 2 µm |
| P. lima + bentonite | 11.7 | 50.6 | 81.5 | 93.7 | 98.2 | 99.4 | 100 | 80% < 2 µm |
| P. lima + kaolinite | 30.1 | 53.2 | 82 | 90.4 | 97.8 | 100 | 100 | 90% < 10 µm |
| P. lima + bentonite Na | 19.8 | 68.4 | 74.9 | 94.2 | 94.2 | 99.4 | 100 | 80% < 70 µm |
3. Experimental Section
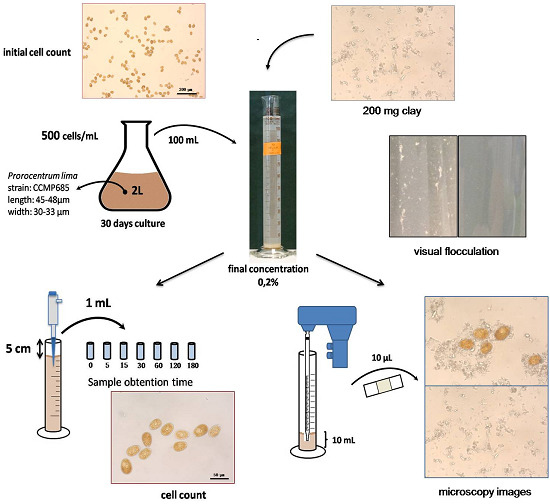
3.1. Prorocentrum lima Culture
3.2. Clays Selection
3.3. Flocculation Assay
3.4. Microscopy Images
3.5. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef] [PubMed]
- Prego-Faraldo, M.V.; Valdiglesias, V.; Mendez, J.; Eirin-Lopez, J.M. Okadaic acid meet and greet: An insight into detection methods, response strategies and genotoxic effects in marine invertebrates. Mar. Drugs 2013, 11, 2829–2845. [Google Scholar] [CrossRef] [PubMed]
- Doblin, M.A.; Dobbs, F.C. Setting a size-exclusion limit to remove toxic dinoflagellate cysts from ships’ ballast water. Mar. Pollut. Bull. 2006, 52, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Burkholder, J.M.; Cochlan, W.P.; Glibert, P.M.; Gobler, C.J.; Heil, C.A.; Kudela, R.; Parsons, M.L.; Rensel, J.E.; Townsend, D.W.; et al. Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae 2008, 8, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, M.; Marvin, H.J.; Kleter, G.A.; Battilani, P.; Brera, C.; Coni, E.; Cubadda, F.; Croci, L.; de Santis, B.; Dekkers, S.; et al. Climate change and food safety: An emerging issue with special focus on Europe. Food Chem. Toxicol. 2009, 47, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Icarus Allen, J.; Artioli, Y.; Beusen, A.; Bouwman, L.; Harle, J.; Holmes, R.; Holt, J. Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: Projections based on model analysis. Glob. Chang. Biol. 2014, 20, 3845–3858. [Google Scholar] [CrossRef] [PubMed]
- Quilliam, M.A. Phycotoxins. J. AOAC Int. 1999, 82, 773–781. [Google Scholar] [PubMed]
- Morono, A.; Arevalo, F.; Fernandez, M.L.; Maneiro, J.; Pazos, Y.; Salgado, C.; Blanco, J. Accumulation and transformation of DSP toxins in mussels Mytilus galloprovincialis during a toxic episode caused by Dinophysis acuminata. Aquat. Toxicol. 2003, 62, 269–280. [Google Scholar] [CrossRef]
- James, K.J.; Carey, B.; O’Halloran, J.; van Pelt, F.N.; Skrabakova, Z. Shellfish toxicity: Human health implications of marine algal toxins. Epidemiol. Infect. 2010, 138, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Bialojan, C.; Takai, A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem. J. 1988, 256, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. The structure and regulation of protein phosphatases. Annu. Rev. Biochem. 1989, 58, 453–508. [Google Scholar] [CrossRef] [PubMed]
- Leira, F.; Alvarez, C.; Vieites, J.M.; Vieytes, M.R.; Botana, L.M. Study of cytoskeletal changes induced by okadaic acid in BE(2)-M17 cells by means of a quantitative fluorimetric microplate assay. Toxicol. in Vitro 2001, 15, 277–282. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Laffon, B.; Pasaro, E.; Mendez, J. Okadaic acid induces morphological changes, apoptosis and cell cycle alterations in different human cell types. J. Environ. Monit. 2011, 13, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Opsahl, J.A.; Ljostveit, S.; Solstad, T.; Risa, K.; Roepstorff, P.; Fladmark, K.E. Identification of dynamic changes in proteins associated with the cellular cytoskeleton after exposure to okadaic acid. Mar. Drugs 2013, 11, 1763–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, A.; Flynn, K.J. Promotion of harmful algal blooms by zooplankton predatory activity. Biol. Lett. 2006, 2, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, D.S.; Jeong, S.Y.; Lee, W.J.; Lee, M.S. Isolation and characterization of a marine algicidal bacterium against the harmful raphidophyceae Chattonella marina. J. Microbiol. 2009, 47, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Zhou, Y.; Zheng, W.; Yu, C.; Zheng, T. Novel insights into the algicidal bacterium DH77-1 killing the toxic dinoflagellate Alexandrium tamarense. Sci. Total Environ. 2014, 482–483, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Herzi, F.; Jean, N.; Zhao, H.; Mounier, S.; Mabrouk, H.H.; Hlaili, A.S. Copper and cadmium effects on growth and extracellular exudation of the marine toxic dinoflagellate Alexandrium catenella: 3D-fluorescence spectroscopy approach. Chemosphere 2013, 93, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zou, H.; Chen, H.; Yuan, X. Removal of harmful cyanobacterial blooms in Taihu Lake using local soils. III. Factors affecting the removal efficiency and an in situ field experiment using chitosan-modified local soils. Environ. Pollut. 2006, 141, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Zhong, J.; Song, L.; Guo, C.; Gan, N.; Wu, Z. Harmful algal bloom removal and eutrophic water remediation by commercial nontoxic polyamine-co-polymeric ferric sulfate-modified soils. Environ. Sci. Pollut. Res. Int. 2015, 22, 10636–10646. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Dai, L.; Li, L.; He, L.; Li, H.; Bi, L.; Gulati, R.D. Reducing the recruitment of sedimented algae and nutrient release into the overlying water using modified soil/sand flocculation-capping in eutrophic lakes. Environ. Sci. Technol. 2012, 46, 5077–5084. [Google Scholar] [CrossRef] [PubMed]
- Sengco, M.R.; Anderson, D.M. Controlling harmful algal blooms through clay flocculation. J. Eukaryot. Microbiol. 2004, 51, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Paineau, E.; Michot, L.J.; Bihannic, I.; Baravian, C. Aqueous suspensions of natural swelling clay minerals. 2. Rheological characterization. Langmuir 2011, 27, 7806–7819. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiao, B.; Li, R.; Wang, C.; Huang, J.; Wang, Z. Mechanisms and factors affecting sorption of microcystins onto natural sediments. Environ. Sci. Technol. 2011, 45, 2641–2647. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, T.C.; Ren, Y. Testosterone sorption and desorption: Effects of soil particle size. J. Hazard. Mater. 2014, 279, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pan, G. A universal method for flocculating harmful algal blooms in marine and fresh waters using modified sand. Environ. Sci. Technol. 2013, 47, 4555–4562. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, J.; Li, Z.; Wang, Y.; Fu, B.; Han, X.; Zheng, L. Cultivation of the benthic microalga Prorocentrum lima for the production of diarrhetic shellfish poisoning toxins in a vertical flat photobioreactor. Bioresour. Technol. 2015, 179, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Casas, L.M.; Legido, J.L.; Pozo, M.; Mourelle, L.; Plantier, F.; Bessieres, D. Specific heat of mixtures of bentonitic clay with sea water or distilled water for their use in thermotherapy. Thermochim. Acta 2011, 524, 68–73. [Google Scholar] [CrossRef]
- Casas, L.M.; Pozo, M.; Gomez, C.P.; Pozo, E.; Bessieres, L.D.; Plantier, F.; Legido, J.L. Thermal behavior of mixtures of bentonitic clay and saline solutions. Appl. Clay Sci. 2013, 72, 18–25. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louzao, M.C.; Abal, P.; Fernández, D.A.; Vieytes, M.R.; Legido, J.L.; Gómez, C.P.; Pais, J.; Botana, L.M. Study of Adsorption and Flocculation Properties of Natural Clays to Remove Prorocentrum lima. Toxins 2015, 7, 3977-3988. https://doi.org/10.3390/toxins7103977
Louzao MC, Abal P, Fernández DA, Vieytes MR, Legido JL, Gómez CP, Pais J, Botana LM. Study of Adsorption and Flocculation Properties of Natural Clays to Remove Prorocentrum lima. Toxins. 2015; 7(10):3977-3988. https://doi.org/10.3390/toxins7103977
Chicago/Turabian StyleLouzao, Maria Carmen, Paula Abal, Diego A. Fernández, Mercedes R. Vieytes, José Luis Legido, Carmen P. Gómez, Jesus Pais, and Luis M. Botana. 2015. "Study of Adsorption and Flocculation Properties of Natural Clays to Remove Prorocentrum lima" Toxins 7, no. 10: 3977-3988. https://doi.org/10.3390/toxins7103977
APA StyleLouzao, M. C., Abal, P., Fernández, D. A., Vieytes, M. R., Legido, J. L., Gómez, C. P., Pais, J., & Botana, L. M. (2015). Study of Adsorption and Flocculation Properties of Natural Clays to Remove Prorocentrum lima. Toxins, 7(10), 3977-3988. https://doi.org/10.3390/toxins7103977