Detoxification of Deoxynivalenol via Glycosylation Represents Novel Insights on Antagonistic Activities of Trichoderma when Confronted with Fusarium graminearum
Abstract
:1. Introduction
2. Results
2.1. Impact of Trichoderma Strains on Growth of F. graminearum 5035 in Dual Culture
2.2. Effect of Trichoderma Strains on Mycotoxin Production of F. graminearum 5035 in Dual Culture
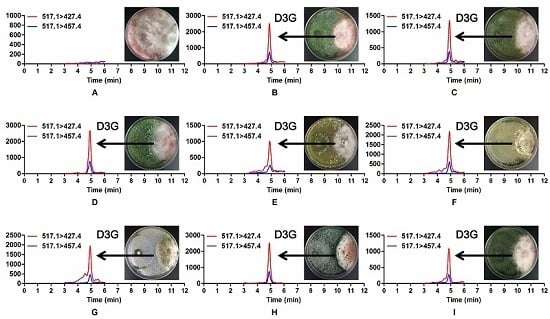
2.3. Occurrence of Modified Mycotoxin D3G in Dual Culture of Trichoderma and F. graminearum
2.4. A Significant Difference in D3G/DON Ratios of Trichoderma Strains when Confronted with F. graminearum 5035
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Standards and Chemicals
5.2. Fungal Strains and Culture Medium
5.3. Antagonistic Activities of Trichoderma on Growth and Mycotoxin Production of F. graminearum
5.4. Fusarium Mycotoxin Extraction and Preparation
5.5. Mycotoxin Determination by LC-MS/MS
5.6. LC-HRMS Analysis of D3G
5.7. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.J.; Mccormick, S.P.; Waalwijk, C.; Lee, T.V.D.; Proctor, R.H. The genetic basis for 3-ADON and 15-ADON trichothecene chemotypes in Fusarium. Fungal Genet. Biol. 2011, 48, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Audenaert, K.; Vanheule, A.; Höfte, M.; Haesaert, G. Deoxynivalenol: A major player in the multifaceted response of Fusarium to its environment. Toxins 2013, 6, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponts, N. Mycotoxins are a component of Fusarium graminearum stress-response system. Front. Microbiol. 2015, 6, 1234. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.-H.; Desjardins, A.; Plattner, R. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia 2002, 153, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glössl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Lemmens, M.; Werner, U.; Krska, R.; Hauser, M.; Adam, G.; Schuhmacher, R. Short review: Metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone in plants. Mycotoxin Res. 2007, 23, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Sundstol Eriksen, G.; Pettersson, H.; Lundh, T. Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food Chem. Toxicol. 2004, 42, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available online: http://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=2947 (accessed on 25 November 2015).
- Broekaert, N.; Devreese, M.; Demeyere, K.; Berthiller, F.; Michlmayr, H.; Varga, E.; Adam, G.; Meyer, E.; Croubels, S. Comparative in vitro cytotoxicity of modified deoxynivalenol on porcine intestinal epithelial cells. Food Chem. Toxicol. 2016, 95, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wegulo, S.N.; Baenziger, P.S.; Hernandez Nopsa, J.; Bockus, W.W.; Hallen-Adams, H. Management of Fusarium head blight of wheat and barley. Crop Prot. 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Yuen, G.Y.; Schoneweis, S.D. Strategies for managing Fusarium head blight and deoxynivalenol accumulation in wheat. Int. J. Food Microbiol. 2007, 119, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tan, Y.; Liu, N.; Liao, Y.; Sun, C.; Wang, S.; Wu, A. Functional agents to biologically control deoxynivalenol contamination in cereal grains. Front. Microbiol. 2016, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Schoneberg, A.; Musa, T.; Voegele, R.T.; Vogelgsang, S. The potential of antagonistic fungi for control of Fusarium graminearum and Fusarium crookwellense varies depending on the experimental approach. J. Appl. Microbiol. 2015, 118, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Selvaraj, J.N.; Xing, F.; Zhou, L.; Wang, Y.; Song, H.; Tan, X.; Sun, L.; Sangare, L.; Folly, Y.M.; et al. Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS ONE 2014, 9, e92486. [Google Scholar] [CrossRef] [PubMed]
- Matarese, F.; Sarrocco, S.; Gruber, S.; Seidl-Seiboth, V.; Vannacci, G. Biocontrol of Fusarium head blight: Interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology 2012, 158, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Malmierca, M.G.; McCormick, S.P.; Cardoza, R.E.; Alexander, N.J.; Monte, E.; Gutierrez, S. Production of trichodiene by Trichoderma harzianum alters the perception of this biocontrol strain by plants and antagonized fungi. Environ. Microbiol. 2015, 17, 2628–2646. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Ann. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Benitez, T.; Rincon, A.M.; Limon, M.C.; Codon, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar] [PubMed]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 1590–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.-P.; Martine, K.-C.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junctions proteins and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Price, N.P.J.; Kurtzman, C.P. Glucosylation and other biotransformations of T-2 toxin by yeasts of the Trichomonascus clade. Appl. Environ. Microb. 2012, 78, 8694–8702. [Google Scholar] [CrossRef] [PubMed]
- Naef, A.; Senatore, M.; Défago, G. A microsatellite based method for quantification of fungi in decomposing plant material elucidates the role of Fusarium graminearum DON production in the saprophytic competition with Trichoderma atroviride in maize tissue microcosms. Fems Microbiol. Ecol. 2006, 55, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Schmeitzl, C.; Warth, B.; Fruhmann, P.; Michlmayr, H.; Malachova, A.; Berthiller, F.; Schuhmacher, R.; Krska, R.; Adam, G. The metabolic fate of deoxynivalenol and its acetylated derivatives in a wheat suspension culture: Identification and detection of DON-15-o-glucoside, 15-acetyl-DON-3-o-glucoside and 15-acetyl-DON-3-sulfate. Toxins 2015, 7, 3112–3126. [Google Scholar] [CrossRef] [PubMed]
- Martina, C.; Silvia, G.; Andrea, D.E.; Pietro, L.; Gianluca, F.; Andrea, M.; Gianni, G.; Chiara, D.A. Durum wheat (Triticum durum Desf.) lines show different abilities to form masked mycotoxins under greenhouse conditions. Toxins 2014, 6, 81–95. [Google Scholar]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Shin, S.; Heinen, S.; Dill-Macky, R.; Berthiller, F.; Nersesian, N.; Clemente, T.; Mccormick, S.; Muehlbauer, G.J. Transgenic wheat expressing a barley UDP-glucosyltransferase detoxifies deoxynivalenol and provides high levels of resistance to Fusarium graminearum. Mol. Plant-Microbe Interact. 2015, 28, 1237–1246. [Google Scholar] [PubMed]
- Shin, S.; Torres-Acosta, J.A.; Heinen, S.J.; McCormick, S.; Lemmens, M.; Paris, M.P.K.; Berthiller, F.; Adam, G.; Muehlbauer, G.J. Transgenic Arabidopsis thaliana expressing a barley UDP-glucosyltransferase exhibit resistance to the mycotoxin deoxynivalenol. J. Exp. Bot. 2012, 63, 4731–4740. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, T.; Young, J.C.; Boland, G.J.; Scott, P.M. Chemical and biological transformations for detoxification of trichothecene mycotoxins in human and animal food chains: A review. Trends Food Sci. Technol. 2010, 21, 67–76. [Google Scholar] [CrossRef]
- Lutz, M.P.; Feichtinger, G.; Defago, G.; Duffy, B. Mycotoxigenic Fusarium and deoxynivalenol production repress chitinase gene expression in the biocontrol agent Trichoderma atroviride P1. Appl. Environ. Microbiol. 2003, 69, 3077–3084. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, R.; Yu, J.; Saravanakumar, K.; Chen, J. Antagonistic and biocontrol potential of Trichoderma asperellum zjsx5003 against the maize stalk rot pathogen Fusarium graminearum. Indian J. Microbiol. 2016, 56, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Iwahashi, Y. Low toxicity of deoxynivalenol-3-glucoside in microbial cells. Toxins (Basel) 2015, 7, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, J.-C.; Changenet, V.; Macadré, C.; Boex-Fontvieille, E.; Soulhat, C.; Bouchabké-Coussa, O.; Dalmais, M.; Atanasova-Pénichon, V.; Bendahmane, A.; Saindrenan, P.; et al. A Brachypodium UDP-glycosytransferase confers root tolerance to deoxynivalenol and resistance to Fusarium infection. Plant Physiol. 2016, 172, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, W.; Pasquet, J.C.; Nussbaumer, T.; Paris, M.P.K.; Wiesenberger, G.; Macadre, C.; Ametz, C.; Berthiller, F.; Lemmens, M.; Saindrenan, P.; et al. Functional characterization of two clusters of Brachypodium distachyon UDP-glycosyltransferases encoding putative deoxynivalenol detoxification genes. Mol. Plant-Microbe Interact. 2013, 26, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, W.; Boddu, J.; Shin, S.; Poppenberger, B.; Berthiller, F.; Lemmens, M.; Muehlbauer, G.J.; Adam, G. Validation of a candidate deoxynivalenol-inactivating UDP-glucosyltransferase from barley by heterologous expression in yeast. Mol. Plant-Microbe Interact. 2010, 23, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Lulin, M.; Yi, S.; Aizhong, C.; Zengjun, Q.; Liping, X.; Peidu, C.; Dajun, L.; Xiu-E, W. Molecular cloning and characterization of an up-regulated UDP-glucosyltransferase gene induced by DON from Triticum aestivum L. cv. Wangshuibai. Mol. Biol. Rep. 2010, 37, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, H.; Malachova, A.; Varga, E.; Kleinova, J.; Lemmens, M.; Newmister, S.; Rayment, I.; Berthiller, F.; Adam, G. Biochemical characterization of a recombinant UDP-glucosyltransferase from rice and enzymatic production of deoxynivalenol-3-O-β-d-glucoside. Toxins 2015, 7, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Tijerino, A.; Elena Cardoza, R.; Moraga, J.; Malmierca, M.G.; Vicente, F.; Aleu, J.; Collado, I.G.; Gutiérrez, S.; Monte, E.; Hermosa, R. Overexpression of the trichodiene synthase gene tri5 increases trichodermin production and antimicrobial activity in Trichoderma brevicompactum. Fungal Genet. Biol. 2011, 48, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Michlmayr, H.; Malachova, A.; Koutnik, A.; Maloku, I.; Berthiller, F.; Adam, G.; Krska, R.; et al. Biotransformation of the mycotoxin deoxynivalenol in Fusarium resistant and susceptible near isogenic wheat lines. PLoS ONE 2015, 10, e0119656. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Fruhmann, P.; Wiesenberger, G.; Kluger, B.; Sarkanj, B.; Lemmens, M.; Hametner, C.; Fröhlich, J.; Adam, G.; Krska, R. Deoxynivalenol-sulfates: Identification and quantification of novel conjugated (masked) mycotoxins in wheat. Anal. Bioanal. Chem. 2015, 407, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.D.; Li, H.P.; Yuan, Q.S.; Song, X.S.; Yao, W.; He, W.J.; Zhang, J.B.; Liao, Y.C. Antagonistic mechanism of iturin a and plipastatin a from Bacillus amyloliquefaciens s76-3 from wheat spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Rao, Q.; Song, S.; Na, L.; Zheng, H.; Hou, J.; Wu, A. Simultaneous determination of major type B trichothecenes and deoxynivalenol-3-glucoside in animal feed and raw materials using improved DSPE combined with LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 963, 75–82. [Google Scholar] [CrossRef] [PubMed]






| Strain Code | Strain | Source |
|---|---|---|
| 5035 | Fusarium graminearum | Huazhong Agricultural University, China |
| JF309 | Trichoderma harzianum | Isolated from Lentinus edodes in our lab |
| GIM3.442 | Trichoderma harzianum | GIMCC, China |
| GIM3.137 | Trichoderma koningii | GIMCC, China |
| GIM3.534 | Trichoderma longibranchiatum | GIMCC, China |
| Q710613 | Trichoderma harzianum | CCCT, SJTU, China |
| Q710251 | Trichoderma atroviride | CCCT, SJTU, China |
| Q710682 | Trichoderma asperellum | CCCT, SJTU, China |
| Q710925 | Trichoderma virens | CCCT, SJTU, China |
| Mycotoxin | Precursor Ion (m/z) | Retention Time (min) | Primary Product Ion (m/z) | Collision Energy (ev) | Secondary Product Ion (m/z) | Collision Energy (ev) |
|---|---|---|---|---|---|---|
| DON | 355.1 [M + Ac]− | 5.04 | 265.0 a | 17 | 247.2 b | 18 |
| D3G | 517.1 [M + Ac]− | 4.89 | 427.4 a | 22 | 457.4 b | 14 |
| 3-ADON | 397.1 [M + Ac]− | 6.54 | 307.1 a | 16 | 173.1 b | 15 |
| 15-ADON | 356.1 [M + NH4]+ | 6.52 | 321.0 a | 15 | 137.0 b | 12 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Tan, Y.; Liu, N.; Yan, Z.; Liao, Y.; Chen, J.; De Saeger, S.; Yang, H.; Zhang, Q.; Wu, A. Detoxification of Deoxynivalenol via Glycosylation Represents Novel Insights on Antagonistic Activities of Trichoderma when Confronted with Fusarium graminearum. Toxins 2016, 8, 335. https://doi.org/10.3390/toxins8110335
Tian Y, Tan Y, Liu N, Yan Z, Liao Y, Chen J, De Saeger S, Yang H, Zhang Q, Wu A. Detoxification of Deoxynivalenol via Glycosylation Represents Novel Insights on Antagonistic Activities of Trichoderma when Confronted with Fusarium graminearum. Toxins. 2016; 8(11):335. https://doi.org/10.3390/toxins8110335
Chicago/Turabian StyleTian, Ye, Yanglan Tan, Na Liu, Zheng Yan, Yucai Liao, Jie Chen, Sarah De Saeger, Hua Yang, Qiaoyan Zhang, and Aibo Wu. 2016. "Detoxification of Deoxynivalenol via Glycosylation Represents Novel Insights on Antagonistic Activities of Trichoderma when Confronted with Fusarium graminearum" Toxins 8, no. 11: 335. https://doi.org/10.3390/toxins8110335
APA StyleTian, Y., Tan, Y., Liu, N., Yan, Z., Liao, Y., Chen, J., De Saeger, S., Yang, H., Zhang, Q., & Wu, A. (2016). Detoxification of Deoxynivalenol via Glycosylation Represents Novel Insights on Antagonistic Activities of Trichoderma when Confronted with Fusarium graminearum. Toxins, 8(11), 335. https://doi.org/10.3390/toxins8110335