Mechanical Property Changes in Breast Cancer Cells Induced by Stimulation with Macrophage Secretions in Vitro
Abstract
:1. Introduction
2. Materials and Methods
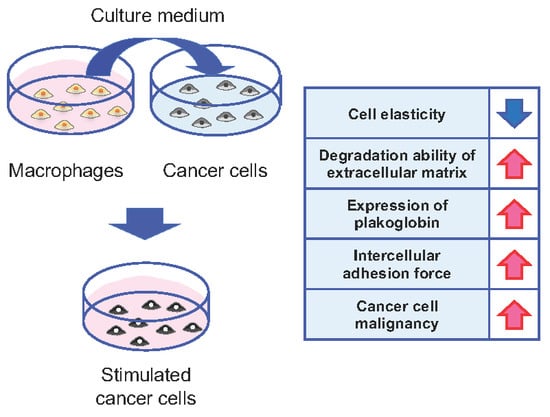
2.1. Cells and the in Vitro Model of Cancer Cell Stimulation
2.2. Measurements of Cell Elasticity
2.3. Measurements of Cell Invasiveness
2.4. Immunofluorescence Assays
2.5. Measurements of Intercellular Adhesion Strengths by AFM
2.6. Data Analysis of AFM Measurements
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Werb, Z.; Lu, P. The role of stroma in tumor development. Cancer J. 2015, 21, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Aras, S.; Zaidi, M.R. TAMeless traitors: macrophages in cancer progression and metastasis. Br. J. Cancer 2017, 117, 1583–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Heusinkveld, M.; van der Burg, S.H. Identification and manipulation of tumor associated macrophages in human cancers. J. Transl. Med. 2011, 9, 216. [Google Scholar] [CrossRef]
- Komohara, Y.; Jinushi, M.; Takeya, M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014, 105, 1–8. [Google Scholar] [CrossRef]
- Komohara, Y.; Niino, D.; Ohnishi, K.; Ohshima, K.; Takeya, M. Role of tumor-associated macrophages in hematological malignancies. Pathol. Int. 2015, 65, 170–176. [Google Scholar] [CrossRef]
- Gordon, S.R.; Aute, R.L.M.; Dulken, B.W.; Hutter, G.; George, B.M.; Ccracken, M.N.M.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Cross, S.E.; Jin, Y.S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef]
- Luo, Q.; Kuang, D.; Zhang, B.; Song, G. Cell stiffness determined by atomic force microscopy and its correlation with cell motility. Biochim. Biophys. Acta 2016, 1860, 1953–1960. [Google Scholar] [CrossRef]
- Kim, H.; Arakawa, H.; Osada, T.; Ikai, A. Quantification of fibronectin and cell surface interactions by AFM. Colloids Surf. B 2002, 25, 33–43. [Google Scholar]
- Kim, H.; Arakawa, H.; Osada, T.; Ikai, A. Quantification of cell adhesion force with AFM: Distribution of vitronectin receptors on a living MC3T3-E1 cell. Ultramicroscopy 2003, 97, 359–363. [Google Scholar] [CrossRef]
- Kim, H.; Arakawa, H.; Hatae, N.; Sugimoto, Y.; Matsumoto, O.; Osada, T.; Ichikawa, A.; Ikai, A. Quantification of the number of EP3 receptors on a living CHO cell surface by the AFM. Ultramicroscopy 2006, 106, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Benoit, M.; Gabriel, D.; Gerisch, G.; Gaub, H.E. Discrete interactions in cell adhesion measured by single-molecule force spectroscopy. Nat. Cell Biol. 2000, 2, 31–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Moy, V.T. Cross-linking of cell surface receptors enhances cooperativity of molecular adhesion. Biophys. J. 2000, 78, 2814–2820. [Google Scholar] [CrossRef]
- Puech, P.H.; Poole, K.; Knebel, D.; Muller, D.J. A new technical approach to quantify cell-cell adhesion forces by AFM. Ultramicroscopy 2006, 106, 637–644. [Google Scholar] [CrossRef]
- Helenius, J.; Heisenberg, C.P.; Gaub, H.E.; Muller, D.J. Single-cell force spectroscopy. J. Cell Sci. 2008, 121, 1785–1791. [Google Scholar] [CrossRef] [Green Version]
- Muller, D.J.; Helenius, J.; Alsteens, D.; Dufrene, Y.F. Force probing surfaces of living cells to molecular resolution. Nat. Chem. Biol. 2009, 5, 383–390. [Google Scholar] [CrossRef]
- Kim, H.; Yamagishi, A.; Imaizumi, M.; Onomura, Y.; Nagasaki, A.; Miyagi, Y.; Okada, T.; Nakamura, C. Quantitative measurements of intercellular adhesion between a macrophage and cancer cells using a cup-attached AFM chip. Colloids Surf. B 2017, 155, 366–372. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Roy, S. Antigen conjugated nanoparticles reprogrammed the tumor-conditioned macrophages toward pro-immunogenic type through regulation of NADPH oxidase and p38MAPK. Cytokine 2019, 113, 162–176. [Google Scholar] [CrossRef]
- Solinas, G.; Schiarea, S.; Liguori, M.; Fabbri, M.; Pesce, S.; Zammataro, L.; Pasqualini, F.; Nebuloni, M.; Chiabrando, C.; Mantovani, A.; et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J. Immunol. 2010, 185, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Ralph, P.; Prichard, J.; Cohn, M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J. Immunol. 1975, 114, 898–905. [Google Scholar] [PubMed]
- Okada, T.; Kurabayashi, A.; Akimitsu, N.; Furihata, M. Expression of cadherin-17 promotes metastasis in a highly bone marrow metastatic murine breast cancer model. Biomed Res. Int. 2017, 2017, 8494286. [Google Scholar] [CrossRef] [PubMed]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Direct measurement of colloidal forces using an atomic force microscope. Nature 1991, 353, 239–241. [Google Scholar] [CrossRef]
- Hertz, H. Ueber die Beruhrung fester eleatischer Korper. J. Reine Angew. Math. 1881, 92, 156–171. [Google Scholar]
- Sneddon, I.N. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int. J. Engng. Sci. 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Haga, H.; Sasaki, S.; Kawabata, K.; Ito, E.; Ushiki, T.; Sambongi, T. Elasticity mapping of living fibroblasts by AFM and immunofluorescence observation of the cytoskeleton. Ultramicroscopy 2000, 82, 253–258. [Google Scholar] [CrossRef]
- Tanaka, A.; Fujii, Y.; Kasai, N.; Okajima, T.; Nakashima, H. Regulation of neuritogenesis in hippocampal neurons using stiffness of extracellular microenvironment. Plos One 2018, 13, e0191928. [Google Scholar] [CrossRef]
- Kim, H.; Ishibashi, K.; Matsuo, K.; Kira, A.; Onomura, Y.; Okada, T.; Nakamura, C. Adhesion strength of a living cell to various substrates measured using a cup-attached atomic force microscopy chip. Jpn. J. Appl. Phys. 2018, 57, 03EB01. [Google Scholar] [CrossRef]
- Kim, H.; Terazono, H.; Takei, H.; Yasuda, K. Cup-shaped superparamagnetic hemispheres for size-selective cell filtration. Sci. Rep. 2014, 4, 6362. [Google Scholar] [CrossRef]
- Fischer-Cripps, A.C. The hertzian contact surface. J. Mater. Sci. 1999, 34, 129. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Terazono, H.; Nakamura, Y.; Sakai, K.; Hattori, A.; Odaka, M.; Girault, M.; Arao, T.; Nishio, K.; Miyagi, Y.; et al. Development of on-chip multi-imaging flow cytometry for identification of imaging biomarkers of clustered circulating tumor cells. Plos One 2014, 9, e104372. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Kenmotsu, H.; Koh, Y.; Yoshino, T.; Yoshikawa, T.; Naito, T.; Takahashi, T.; Murakami, H.; Nakamura, Y.; Tsuya, A.; et al. Size-based isolation of circulating tumor cells in lung cancer patients using a microcavity array system. Plos One 2013, 8, e67466. [Google Scholar] [CrossRef] [PubMed]
- El-Kirat-Chatel, S.; Dufrene, Y.F. Nanoscale adhesion forces between the fungal pathogen Candida albicans and macrophages. Nanoscale Horiz. 2016, 1, 69–74. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Ishibashi, K.; Okada, T.; Nakamura, C. Mechanical Property Changes in Breast Cancer Cells Induced by Stimulation with Macrophage Secretions in Vitro. Micromachines 2019, 10, 738. https://doi.org/10.3390/mi10110738
Kim H, Ishibashi K, Okada T, Nakamura C. Mechanical Property Changes in Breast Cancer Cells Induced by Stimulation with Macrophage Secretions in Vitro. Micromachines. 2019; 10(11):738. https://doi.org/10.3390/mi10110738
Chicago/Turabian StyleKim, Hyonchol, Kenta Ishibashi, Tomoko Okada, and Chikashi Nakamura. 2019. "Mechanical Property Changes in Breast Cancer Cells Induced by Stimulation with Macrophage Secretions in Vitro" Micromachines 10, no. 11: 738. https://doi.org/10.3390/mi10110738
APA StyleKim, H., Ishibashi, K., Okada, T., & Nakamura, C. (2019). Mechanical Property Changes in Breast Cancer Cells Induced by Stimulation with Macrophage Secretions in Vitro. Micromachines, 10(11), 738. https://doi.org/10.3390/mi10110738