1. Introduction
Formaldehyde is a major pollutant of indoor air due to its multiple sources (materials, combustion, painting, etc.). Several studies [
1,
2,
3,
4] have shown that indoor formaldehyde concentrations are 2–15 times higher than those measured outdoors, and may vary typically between 10 to 100 μg/m
3. Casset et al. have shown that formaldehyde is implicated in allergic diseases, particularly for asthmatic people [
5]. Starting from 2004, formaldehyde is considered as a carcinogenic compound for humans by the International Agency for Research on Cancer [
6]. Consequently, current French regulations recommend a limit of 30 μg/m
3 for chronic exposure and 50 μg/m
3 for acute exposure [
7] in order to reduce the health impact.
The main analytical methods allowing for the quantification of airborne formaldehyde concentrations uses an active or passive sampling on a 2,4-dinitrophenylhydrazine (DNPH) tube for several hours or several days, respectively, followed by an HPLC/UV analysis, which is time-consuming and involves a laboratory treatment with bulky instruments. In addition, this approach does not provide real-time results and only gives an average value of the concentration.
Many alternative methods oriented toward real-time and in-field detection have been developed, such as the Hantzsch monitor, proton-transfer-reaction mass spectrometry (PTR-MS), GC-MS, and infrared diode laser spectroscopy [
8,
9,
10,
11]. However, most of these methods rely on bulky apparatuses, and as such, are not adapted for indoor air monitoring where portability is a key point. Among the methods previously developed, some use specific reagents that can react with formaldehyde to produce a compound that can be detected, either via colorimetry or fluorescence [
12,
13,
14]. Miniature and portable systems based on a color change in solid materials, which is conveniently used for the detection, have been developed [
15,
16]. Nevertheless, autonomic and fully continuous monitoring was not achieved since with time, the solid detection element got saturated and could not be renewed without human intervention.
Even if measuring 30 or 50 μg/m3 is not particularly complicated from an analytical point of view, it is more challenging to perform it in real-time by means of a sufficiently precise and rapid apparatus suitable for indoor environments, i.e., small, portable, with a low operation noise, and running on a battery. Most of the near real-time sensitive and precise techniques available on the market run on mains power and are not portable, except for the instruments marketed by Ethera (Nemo), more recently by In’Air Solutions (In’Air µF-1), and probably a few other manufacturers. However, none combines satisfactory portability, a small footprint, battery operation, being silent, a time resolution lower than 1 min, and a detection limit better than 1 µg/m3. Usually, the real-time techniques use a gas pump and a mass flow controller to perform the sampling, making them particularly costly, bulky, and energy intensive, which is a strong limitation for a portable instrument.
The main goal of this work was to solve these issues and thus to develop a formaldehyde analytical method and the associated laboratory prototype in order to quantify gaseous formaldehyde concentrations in near-real time. The technique was based on three sequential steps:
Passive gaseous formaldehyde uptake into an aqueous acetylacetone solution through a microporous membrane;
The derivatization reaction at 65 °C to quantitatively convert formaldehyde into an easily detectable fluorescent molecule;
The detection of 3,5-diacetyl-1,4-dihydrolutidine (DDL) using fluorescence.
The gaseous formaldehyde uptake efficiency has been optimized under controlled laboratory conditions by varying the microporous tube length, the liquid flow rate inside the tube, the gas flow rate applied close to the porous interface, and the gaseous formaldehyde concentration. Finally, the linearity and the sensitivity of the microdevice have been evaluated.
3. Results and Discussion
The uptake efficiency of the gaseous formaldehyde was evaluated and optimized under laboratory conditions by varying the microporous tube length, the liquid flow rate inside the tube, the gas flow rate injected around the microporous tube housing, and the gaseous formaldehyde concentration. The results are detailed and then discussed in the sections below.
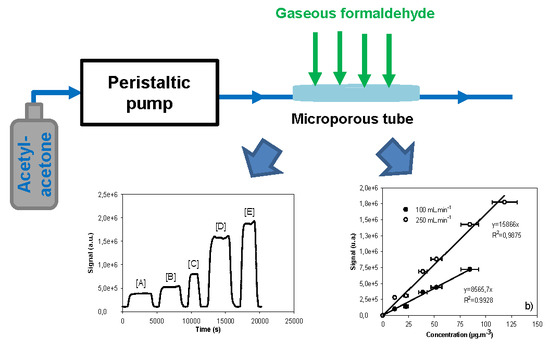
Figure 3 shows some typical fluorescence signals obtained with various gaseous formaldehyde concentrations ranging between 12 and 118 µg/m3 for given liquid and gas flow rates of 17 µL/min and 250 NmL/min, respectively. With the photomultiplier gain set at 40%, the fluorescence signal remained below the saturation value of 2.1 × 106 a.u., regardless of the formaldehyde concentrations. Prior to each measurement performed with a known gas concentration of formaldehyde, pure air was injected around the microporous tube to obtain a reference signal called the baseline or blank. The joint use of pure air and pure acetyl acetone solution free of formaldehyde for a few minutes allowed for a quick renewal of the interface and purge of the entire device. The fluorescence signal then fell back to its baseline level. No significant baseline deviation was observed during the duration of the experiments. The net average height of the fluorescence signal, corresponding to the formaldehyde concentration, was calculated by subtracting the baseline value from the raw height of the fluorescence signal. The error on this resulting signal was calculated to be two times the standard deviation.
3.1. Influence of the Microporous Tube Length
The geometry and the size of the porous membrane can strongly affect the gas uptake, since both modify the total area of the interface. Here, the effect of the microporous tube length was studied under the same experimental conditions in the range 3.2–27.5 cm with 7 tubes, i.e., 3.2, 5.3, 7.5, 10.5, 13.0, 19.5, and 27.5 cm. The formaldehyde concentrations were set to 48.0 μg/m3 (39.3 ppb), corresponding to a medium indoor air level. Gas and liquid flow rates were fixed to 250 NmL/min and 17 µL/min, respectively.
As shown in
Figure 4, the fluorescence signal increased linearly with the microporous tube length up to 27.5 cm. Such an observation supported the assumption of the establishment of a dynamic equilibrium between the gas and liquid phases, where gas–liquid contact time influenced the gas uptake yield. This optimization tended to show that porous tube length could be modified according to the targeted concentration ranges measured, where a shorter tube could be used for highly polluted environments. Conversely, a longer tube will be better suited to quantify low airborne formaldehyde levels but will increase the response time of the analytical instrument.
In the framework of this study, the length of 10.0 cm seemed to be a good compromise to trap a significant amount of formaldehyde into the aqueous solution of acetylacetone without excessively increasing the response time of the instrument. Indeed, the internal volume of this porous tube was about 25.4 µL/cm, implying a renewal time of about 15 min at 17 µL/min with a 10-cm tube length. Consequently, this length was chosen for the following experiments.
3.2. Influence of the Liquid Flow Rate
The liquid flow rate may influence the “pumping” effect through the porous membrane, modifying the gas uptake efficiency. This potential effect was investigated under the same experimental conditions by varying the liquid flow rate in the range 9–33 µL/min. Based on the results detailed in the previous section, the microporous tube length was fixed to 10.0 cm. The gas flow rate was equal to 250 NmL/min, while the gaseous formaldehyde concentration was 48.0 µg/m3 (39.3 ppb).
The results are displayed in
Figure 5 where it is observed that the fluorescence signal decreased significantly when the liquid flow rate varied from 9 to 33 µL/min. In this range, the liquid flow rate was always sufficiently low to have a sufficient residential time in the oven to fully convert formaldehyde into DDL. Similarly, for the observations made for the influence of the microporous tube length, these experimental results confirmed that a dynamic equilibrium between gas and liquid phases was established and strongly depended on the gas–liquid contact time, which thus drove the efficiency of the gaseous formaldehyde uptake into the aqueous phase. In order to simultaneously minimize the response time and limit the reagent consumption, the liquid flow rate was fixed at 17 µL/min for the following experiments, with this value being a compromise between a rapid analytical response, an enhanced signal of fluorescence, and an acceptable reagent consumption.
3.3. Influence of the Gas Flow Rate
The gas flow rate could modify the gaseous formaldehyde concentration at the porous interface by avoiding the scarcity of formaldehyde molecules near the exchange porous surface, which could be due to the rapid mass transfer to the aqueous solution. For this reason, the gas flow rate was varied in the range 50–300 NmL/min, whereas all the other parameters were maintained as follows: microporous tube length of 10.0 cm, liquid flow rate of 17 µL/min, and gaseous formaldehyde concentration of 84.6 µg/m3.
Figure 6 presents the results obtained and indicates that the fluorescence signal, and thus the formaldehyde uptake efficiency, strongly increased from 4.8 × 10
5 to 11.1 × 10
5 a.u. when the gas flow rate went up from 50 to 300 NmL/min. However, a plateau was not reached, suggesting that higher flow rates could improve the gas transfer until the point where the gas phase concentration near the porous surface would be kept constant such that the renewal of the gas mixture at the interface could be the kinetically limiting process while its uptake into the liquid phase itself where the gas goes through the membrane appears to be fast enough.
3.4. Influence of Gaseous Formaldehyde Concentrations
This section demonstrates that the fluorescence signal was proportional to the gaseous concentration of formaldehyde when the experimental conditions were optimal. Indeed, an analytical device needs to be calibrated in the optimized conditions to give relevant measurements in the field.
Regarding the potential impact of the gas flow rate on the uptake efficiency, as demonstrated in the previous section, several calibrations curves were performed at different gas flow rates, i.e., 20, 60, 100, and 250 NmL/min. The gaseous formaldehyde concentrations varied in the range 0–118 µg/m3.
For all the gas flow rates investigated, the fluorescence signal increased linearly with the increase of the gaseous formaldehyde concentration, as displayed in
Figure 7. This indicated that the formaldehyde amount trapped into the solution was perfectly proportional to that in the gas phase, regardless of the gas concentration and the gas flow rate. However, the slope of the linear plot increased from 1875 to 15,866 m
3/μg when the gas flow rate varied between 20 and 250 mL/min. Even when comparing the highest gas flow rates, the slope of the linear fit at 100 NmL/min was approximately 40% lower than that obtained at 250 NmL/min.
A higher flow rate avoided the rarefaction of formaldehyde molecules near the microporous interface and minimized the establishment of a concentration gradient by quickly regenerating the gaseous mixture close to the interface. These results demonstrated that it was necessary to have an effective renewal of the gas concentration near the gas–liquid interface, regardless of the technical solution used, i.e., the use of a gas pump or a set of efficient fans.
3.5. Analytical Performances
From the linear calibrations obtained at the highest gas flow rates of 100 and 250 NmL/min, the sensitivity of the microdevice could be evaluated from the signal intensity obtained at the lowest gaseous formaldehyde concentrations investigated, i.e., 11.2 (9.1 ppb) and 11.7 µg/m3 (9.5 ppb), respectively, and the experimental determination of the signal-to-noise ratio (S/N). Considering that the optimal gas flow rate for the gaseous formaldehyde uptake was 250 mL/min, the theoretical detection and quantification limits were estimated to be 0.13 μg/m3 (0.11 ppb) (S/N = 3) and 0.43 μg/m3 (0.35 ppb) (S/N = 10), respectively. The analytical prototype was slightly less sensitive at 100 mL/min, with values of 0.22 and 0.73 μg/m3 for the limits of detection and quantification, respectively.
In addition, the temporal resolution of 2 s and the response time of 15 min with a 10-cm microporous tube length allowed for the near real-time monitoring of airborne formaldehyde.
Furthermore, the experiments were carried out for 6 months with the same microporous tube sample that was 10 cm in length, showing that its lifetime was satisfactory.
3.6. Preliminary Integration in a Box Equipped with Fans
The possibility to replace the gas pump with fans was tested by placing the microporous tube in a box (20 cm × 25 cm × 15 cm) equipped with two fans sucking the ambient air in at the front, and two grids on the back sides to ensure the air extraction. The microporous tube was placed horizontally, and therefore vertically to the air stream generated, but not in a near proximity to the fans.
According to the observations made in the present work, the fluorescence signal increased depending on the environment in which the prototype was placed. Thus, with clean air, the signal was at the baseline level, i.e., around 46,800 µV, where this signal was obtained with an enhanced version of the data acquisition. In outdoor air, with a concentration of formaldehyde close to 6 μg/m3, the signal was already significantly greater and reached 95,000 µV (8030 µV m3/µg after subtracting the background). In polluted and confined indoor air where the formaldehyde level was of the order of 40 μg/m3, the signal increased again and reached 325,000 µV, i.e. 6960 µV m3/µg once the background was subtracted.
If the signals were perfectly stable, the signals were not found to be fully proportional to the gas phase concentration. From the signal measured at 6 µg/m3, and considering a noise of about 1000 µV, the detection limit was only of the order of 0.4 μg/m3. This lower sensitivity and nonlinear response could be explained using two main reasons:
The selected fans were undersized; ideally, the linear gas velocities achieved should be almost identical to those used earlier in this study.
The air flow around the microporous tube needed to be optimized by modeling, which could determine the optimal design for optimal air renewal near the microporous tube.
4. Conclusions
In this work, a microdevice was developed and the key parameters were optimized to obtain a linear response as a function of the gaseous formaldehyde concentration in the range 10–120 µg/m3, representative of the concentrations measured in indoor air.
The results obtained demonstrate the rarefaction of gaseous formaldehyde concentration at the microporous interface and the establishment of a concentration gradient due to the uptake into the solution. This key point needs to be considered for further prototype enhancement. Indeed, at a low turnover of the gas mixture close to the interface, there was clearly a kinetic limitation to an efficient gas–liquid transfer that could be explained by a slower gas diffusion compared to the uptake process itself. At a higher turnover of the gas mixture, this effect became limited.
This new analytical method allowed for the quantification of formaldehyde in near real-time. With a theoretical limit of detection (LOD) equal to 0.13–0.4 μg/m
3, its very good sensitivity was enough to measure typical concentrations in both indoor and outdoor air. This LOD was also consistent with the upcoming French regulation limiting formaldehyde levels at 10 µg/m
3 in 2023 in indoor air [
7].
In the future, the new microdevice could operate in a fully passive sampling mode if the pump and the housing are removed and replaced by a set of fans to ensure the renewal of the gas mixture around the microporous interface at a sufficient gas flow rate and a subsequent gas velocity. Indeed, if the preliminary tests performed with two fans to replace the set of a costly mass flow controller and the noisy gas pump are encouraging, then further improvements are possible. With the help of modelling, such an implementation could lead to a reliable, quieter, and less-expensive device that is suitable for the monitoring of indoor air quality at a large-scale.