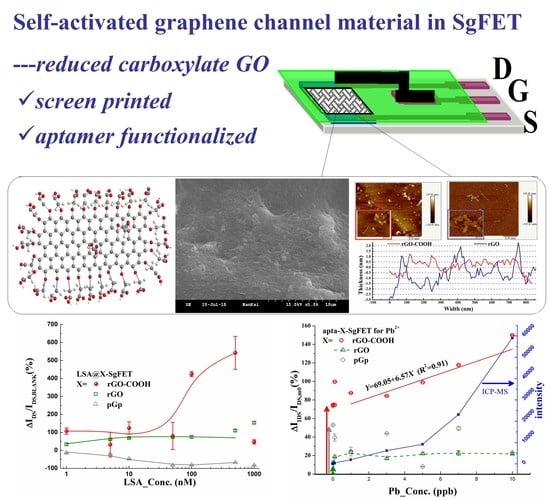
Reduced Carboxylate Graphene Oxide based Field Effect Transistor as Pb2+ Aptamer Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods and Principle for rGO-COOH Preparation
2.3. Methods of Device Fabrication
2.4. Apparatus
2.5. Electronic Set-Up and Measurements for SP-X-FET
2.6. Detection of Pb2+ Standard Samples
2.7. Real Sample Examinations
3. Results and Discussion
3.1. Identification and Morphology
3.2. XPS Characterizations
3.3. Electronic Features
3.4. LSA Immobilization and Pb2+ Detection
3.5. Selectivity and Real Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Green, N.S.; Norton, M.L. Interactions of DNA with graphene and sensing applications of graphene field-effect transistor devices: A review. Anal. Chim. Acta 2015, 853, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.P.; Pham, T.T.T.; Wolfrum, B.; Offenhäusser, A.; Thierry, B. CMOS-Compatible Silicon Nanowire Field-Effect Transistor Biosensor: Technology Development toward Commercialization. Materials 2018, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Kaisti, M. Detection principles of biological and chemical FET sensors. Biosens. Bioelectron. 2017, 98, 437–448. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Farzin, L.; Shamsipur, M.; Sheibani, S. A review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals. Talanta 2017, 174, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, X.; Zhou, Y.; Luo, L.; Zhang, J.; Huang, A.; Mao, Q.; Chen, X.; Tang, L. Aptamer-based biosensors for detection of lead (II) ion: A review. Anal. Methods 2017, 9, 1976–1990. [Google Scholar] [CrossRef]
- Lin, T.C.; Li, Y.S.; Chiang, W.H.; Pei, Z. A high sensitivity field effect transistor biosensor for methylene blue detection utilize graphene oxide nanoribbon. Biosens. Bioelectron. 2017, 89, 511–517. [Google Scholar] [CrossRef]
- Kim, S.G.; Lee, J.S.; Jun, J.; Shin, D.H.; Jang, J. Ultrasensitive Bisphenol A Field-Effect Transistor Sensor Using an Aptamer-Modified Multichannel Carbon Nanofiber Transducer. ACS Appl. Mater. Interfaces 2016, 8, 6602–6610. [Google Scholar] [CrossRef]
- So, H.M.; Park, D.W.; Jeon, E.K.; Kim, Y.H.; Kim, B.S.; Lee, C.K.; Choi, S.Y.; Kim, S.C.; Chang, H.; Lee, J.O. Detection and titer estimation of Escherichia coli using aptamer-functionalized single-walled carbon-nanotube field-effect transistors. Small 2008, 4, 197–201. [Google Scholar] [CrossRef]
- Ohno, Y.; Maehashi, K.; Matsumoto, K. Label-free biosensors based on aptamer-modified graphene field-effect transistors. J. Am. Chem. Soc. 2010, 132, 18012–18013. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cui, X.; Li, Y.; Li, H.; Huang, L.; Bi, J.; Luo, J.; Ma, L.Q.; Zhou, W.; Cao, Y.; et al. A label-free and portable graphene FET aptasensor for children blood lead detection. Sci. Rep. 2016, 6, 21711. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Park, S.J.; Hong, J.Y.; Han, A.R.; Lee, J.S.; Lee, J.S.; Oh, J.H.; Jang, J. Flexible FET-Type VEGF Aptasensor Based on Nitrogen-Doped Graphene Converted from Conducting Polymer. ACS Nano 2012, 6, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Park, S.J.; Kwon, O.S.; Bae, J.; Jang, J. High-performance flexible graphene aptasensor for mercury detection in mussels. ACS Nano 2013, 7, 10563–10571. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Dai, Z.; Tang, X.; Lin, Z.; Lo, P.K.; Meyyappan, M.; Lai, K.W. Biosensing: Graphene Field-Effect Transistors for the Sensitive and Selective Detection of Escherichia coli Using Pyrene-Tagged DNA Aptamer. Adv. Healthc. Mater. 2017, 6, 1700736. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhu, Y.; Wang, X.; Rotti, P.G.; DiMarco, C.; Tyler, S.R.; Zhao, X.; Engelhardt, J.F.; Hone, J.; Lin, Q. Real-Time Monitoring of Insulin Using a Graphene Field-Effect Transistor Aptameric Nanosensor. ACS Appl. Mater. Interfaces 2017, 9, 27504. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.S.; Salimi, A. Ultrasensitive flexible FET-type aptasensor for CA 125 cancer marker detection based on carboxylated multiwalled carbon nanotubes immobilized onto reduced graphene oxide film. Anal. Chim. Acta 2018, 1000, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ju, C.; Li, Y.; Shang, Z.; Wu, Y.; Jia, Y.; Niu, Y. Detection of circulating tumor cells in prostate cancer based on carboxylated graphene oxide modified light addressable potentiometric sensor. Biosens. Bioelectron. 2015, 66, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Sohn, I.Y.; Kim, D.J.; Jung, J.H.; Yoon, O.J.; Thanh, T.N.; Quang, T.T.; Lee, N.E. pH sensing characteristics and biosensing application of solution-gated reduced graphene oxide field-effect transistors. Biosens. Bioelectron. 2013, 45, 70–76. [Google Scholar] [CrossRef]
- Tudorache, M.; Bala, C. Biosensors based on screen-printing technology, and their applications in environmental and food analysis. Anal. Bioanal. Chem. 2007, 388, 565–578. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Martínez-Domingo, C.; Ramon, E.; Merkoçi, A. An Inkjet-Printed Field-Effect Transistor for Label-Free Biosensing. Adv. Funct. Mater. 2014, 24, 6291–6302. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F. Graphene-based screen-printed electrochemical (bio)sensors and their applications: Efforts and criticisms. Biosens. Bioelectron. 2017, 89, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Schirnding, Y.E.; Prapamontol, T. Environmental lead exposure: A public health problem of global dimensions. Bull. World Health Organ. 2000, 78, 1068–1077. [Google Scholar] [PubMed]
- Senhauser, D.A. Minuteman disease: Lead poisoning. Arch. Environ. Health Int. J. 1965, 10, 801–804. [Google Scholar] [CrossRef]
- Assi, M.A.; Hezmee, M.N.M.; Haron, A.W.; Sabri, M.Y.M.; Rajion, M.A. The detrimental effects of lead on human and animal health. Vet. World 2016, 9, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Pelclová, D.; St’astná, J.; Vlcková, S.; VIcek, K.; Urban, M.; Lastovková, A.; DoIezel, Z. Is central Europe safe from environmental lead intoxications? A case series. Cent. Eur. J. Public Health 2016, 24, 120–122. [Google Scholar] [CrossRef]
- Sabbar, M.; Delaville, C.; Deurwaerdère, P.D.; Lakhdar-Ghazal, N.; Benazzouz, A. Lead-Induced Atypical Parkinsonism in Rats: Behavioral, Electrophysiological, and Neurochemical Evidence for a Role of Noradrenaline Depletion. Front. Neurosci. 2018, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ni, Z.M.; Ye, L.X.; Chen, J.W.; Wang, Q.; Zhou, Y.K. Dose-response relationship between blood lead levels and hematological parameters in children from central China. Environ. Res. 2018, 164, 501–506. [Google Scholar] [CrossRef]
- Gibson, S.L.M.; Goldberg, A. Defects in haem synthesis in mammalian tissues in experimental lead poisoning and experimental porphyria. Clin. Sci. 1970, 38, 63–72. [Google Scholar] [CrossRef]
- Jouhadi, Z.; Bensabbahia, D.; Chafiq, F.; Oukkache, B.; Guebessi, N.B.; Abdellah, E.A.; Najib, J. Lead poisoning in children: A case report. Pan Afr. Med. J. 2016, 24, 316. [Google Scholar] [CrossRef]
- Matović, V.; Buh, A.; Ðukić-Ćosi, D.; Bula, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef]
- Choudhury, R.; Misra, T.K. Gluconate Stabilized Silver Nanoparticles as a Colorimetric Sensor for Pb2+. Colloid Surf. 2018, 545, 179–187. [Google Scholar] [CrossRef]
- Kuang, H.; Xing, C.; Hao, C.; Liu, L.; Wang, L.; Xu, C. Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors 2013, 13, 4214–4224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ni, Y.; Kokot, S. The use of DNA self-assembled gold nano-rods for novel analysis of lead and/or mercury in drinking water. Anal. Methods 2015, 13, 4514–4520. [Google Scholar] [CrossRef]
- Jian, J.M.; Liu, Y.Y.; Zhang, Y.L.; Guo, X.S.; Cai, Q. Fast and sensitive detection of Pb2+ in foods using disposable screen-printed electrode modified by reduced graphene oxide. Sensors 2013, 13, 13063–13075. [Google Scholar] [CrossRef] [PubMed]
- Seenivasan, R.; Chang, W.J.; Gunasekaran, S. Highly sensitive detection and removal of lead ions in water using cysteine-functionalized graphene oxide/polypyrrole nanocomposite film electrode. ACS Appl. Mater. Interfaces 2015, 7, 15935–15943. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, F. Studies of functional nucleic acids modified light addressable potentiometric sensors: XPS, biochemical assay and simulation. Anal. Chem. 2018, 90, 5153–5161. [Google Scholar] [CrossRef]
- Xiang, D.; Shigdar, S.; Qiao, G.; Wang, T.; Kouzani, A.Z.; Zhou, S.F.; Kong, L.; Li, Y.; Pu, C.; Duan, W. Nucleic Acid Aptamer-Guided Cancer Therapeutics and Diagnostics: The Next Generation of Cancer Medicine. Theranostics 2015, 5, 23–42. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, T.; Choi, J.; Park, J.; Kim, Y.S.; Chang, M.S.; Jung, H.; Park, K.T.; Yang, S.J.; Park, C.R. Hidden second oxidation step of Hummers method. Chem. Mater. 2016, 28, 756–764. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Garlapati, S.K.; Divya, M.; Breitung, B.; Kruk, R.; Hahn, H.; Dasgupta, S. Printed Electronics Based on Inorganic Semiconductors: From Processes and Materials to Devices. Adv. Mater. 2018, 30, 1707600. [Google Scholar] [CrossRef]
- Arao, Y.; Mori, F.; Kubouchi, M. Efficient solvent systems for improving production of few-layer graphene in liquid phase exfoliation. Carbon 2017, 118, 18–24. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. TI Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, A.; Felten, A.; Verzhbitskiy, I.; Davey, R.; Casiraghi, C. Raman study on defective graphene: Effect of the excitation energy, type, and amount of defects. Phys. Rev. B 2013, 88, 035426. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A. Visualization of defect densities in reduced graphene oxide. Carbon 2012, 50, 3666–3673. [Google Scholar] [CrossRef]
- Eigler, S.; Grimm, S.; Enzelberger-Heim, M.; Mueller, P.; Hirsch, A. Graphene oxide: Efficiency of reducing agents. Chem. Commun. 2013, 49, 7391–7393. [Google Scholar] [CrossRef] [PubMed]
- Bayle, M.; Reckinger, N.; Huntzinger, J.; Felten, A.; Bakaraki, A.; Landois, P.; Paillet, M. Dependence of the Raman spectrum characteristics on the number of layers and stacking orientation in few-layer graphene. Phys. Status Solidi 2015, 252, 2375–2379. [Google Scholar] [CrossRef]
- Ni, Z.H.; Yu, T.; Lu, Y.H.; Feng, Y.P.; Shen, Z.X. Uniaxial strain on graphene: Raman spectroscopy study and band-gap opening. ACS Nano 2008, 2, 2301–2305. [Google Scholar] [CrossRef]
- Tien, H.W.; Huang, Y.L.; Yang, S.Y.; Wang, J.Y.; Ma, C.C. The production of graphene nanosheets decorated with silver nanoparticles for use in transparent, conductive films. Carbon 2011, 49, 1550–1560. [Google Scholar] [CrossRef]
- Kummer, K.; Vyalikh, D.V.; Gavrila, G. Electronic structure of genomic DNA: A photoemission and X-ray absorption study. J. Phys. Chem. B 2010, 114, 9645–9652. [Google Scholar] [CrossRef]
- Gomes, P.J.; Ferraria, A.M.; Botelho do Rego, A.M.; Hoffmann, S.V.; Ribeiro, P.A.; Raposo, M. Energy thresholds of DNA damage induced by UV radiation: An XPS study. J. Phys. Chem. B 2015, 119, 5404–5411. [Google Scholar] [CrossRef]
- Raymundo-Pinero, E.; Cazorla-Amorós, D.; Linares-Solano, A.; Wild, U.; Schlögl, R. Structural characterization of N-containing activated carbon fibers prepared from a low softening point petroleum pitch and a melamine resin. Carbon 2002, 40, 597–608. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, S.J.; Kim, J.K. Preparation of graphite nanoplatelets and graphene sheets. J. Colloid Interface Sci. 2009, 336, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jia, Y. Graphene solution-gated field effect transistor DNA sensor fabricated by liquid exfoliation and double glutaraldehyde cross-linking. Carbon 2018, 130, 758–767. [Google Scholar] [CrossRef]
- Yates, D.E.; Levine, S.; Healy, T.W. Site-binding model of the electrical double layer at the oxide/water interface. J. Chem. Soc. Faraday Trans. 1 1974, 78, 1807. [Google Scholar] [CrossRef]
- Martinoia, S.; Massobrio, G.; Lorenzelli, L. Modeling ISFET microsensor and ISFET-based microsystems: A review. Sens. Actuators B 2005, 105, 14–27. [Google Scholar] [CrossRef]
- Kundu, S.; Pyne, A.; Dutta, R.; Sarkar, N. Unveiling the Interaction of Duplex DNA with Graphene Oxide in the Presence of Two Diverse Binders: A Detailed Photophysical Study. J. Phys. Chem. C 2018, 122, 6876–6888. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Wang, Z.; Jia, Y. Reduced Carboxylate Graphene Oxide based Field Effect Transistor as Pb2+ Aptamer Sensor. Micromachines 2019, 10, 388. https://doi.org/10.3390/mi10060388
Li F, Wang Z, Jia Y. Reduced Carboxylate Graphene Oxide based Field Effect Transistor as Pb2+ Aptamer Sensor. Micromachines. 2019; 10(6):388. https://doi.org/10.3390/mi10060388
Chicago/Turabian StyleLi, Fang, Zhongrong Wang, and Yunfang Jia. 2019. "Reduced Carboxylate Graphene Oxide based Field Effect Transistor as Pb2+ Aptamer Sensor" Micromachines 10, no. 6: 388. https://doi.org/10.3390/mi10060388
APA StyleLi, F., Wang, Z., & Jia, Y. (2019). Reduced Carboxylate Graphene Oxide based Field Effect Transistor as Pb2+ Aptamer Sensor. Micromachines, 10(6), 388. https://doi.org/10.3390/mi10060388