Fabrication of Tapered 3D Microstructure Arrays Using Dual-Exposure Lithography (DEL)
Abstract
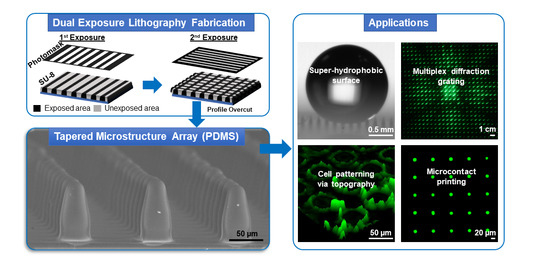
:1. Introduction
2. Materials and Methods
2.1. Photomask Design
2.2. Fabrication of SU-8 Master
2.3. PDMS-MSA Fabrication
2.4. MSA Structural Analysis
2.5. Surface Hydrophobicity Analysis
2.6. Angle-Resolved Far-Field Diffraction Analysis
Intensity Profiling
2.7. Cell Seeding and Imaging on MSA
2.7.1. Coating of PDMS and Fibroblasts Seeding
2.7.2. Cell imaging and Analysis
2.8. Microcontact Printing
2.9. Statistical Analysis
3. Results and Discussion
3.1. Size and Shape Tunability of the MSAs
3.2. Dimensional Effect of the MSAs on Surface Hydrophobicity
3.3. Optical Diffraction Application of the MSA
3.4. MSA as Cell Culturing Substrates
3.5. Protein Patterning through Microcontact Printing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Zhao, J.; Liu, L.; Xue, W. Dimensional effects of polymer pillar arrays on hydrophobicity. Surf. Eng. 2016, 32, 125–131. [Google Scholar] [CrossRef]
- Jin, C.; Qiao, Q. Deformation of Pyramidal PDMS Stamps during Microcontact Printing. J. Appl. Mech. 2016, 83, 71011. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-J.; Mohseni, K.; Bright, V.M. Fabrication of tapered SU-8 structure and effect of sidewall angle for a variable focus microlens using EWOD. Sens. Actuators Phys. 2007, 136, 546–553. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, W.-S.; Lee, K.-H.; Yoon, J.-B. A simple and effective fabrication method for various 3D microstructures: Backside 3D diffuser lithography. J. Micromech. Microeng. 2008, 18, 125015. [Google Scholar] [CrossRef]
- Evans, C.J.; Bryan, J.B. “Structured”, “textured” or “engineered” surfaces. Cirp Ann. 1999, 48, 541–556. [Google Scholar] [CrossRef]
- Liao, X.; Brown, K.A.; Schmucker, A.L.; Liu, G.; He, S.; Shim, W.; Mirkin, C.A. Desktop nanofabrication with massively multiplexed beam pen lithography. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Altuna, A.; Gabriel, G.; de la Prida, L.M.; Tijero, M.; Guimerá, A.; Berganzo, J.; Salido, R.; Villa, R.; Fernández, L.J. SU-8-based microneedles for in vitro neural applications. J. Micromech. Microeng. 2010, 20, 64014. [Google Scholar] [CrossRef] [Green Version]
- Kuo, J.-N.; Hsieh, C.-C.; Yang, S.-Y.; Lee, G.-B. An SU-8 microlens array fabricated by soft replica molding for cell counting applications. J. Micromech. Microeng. 2007, 17, 693. [Google Scholar] [CrossRef]
- Li, H.; Chen, F.; Biria, S.; Hosein, I.D. Prototyping of Superhydrophobic Surfaces from Structure-Tunable Micropillar Arrays Using Visible Light Photocuring. Adv. Eng. Mater. 2019, 21, 1801150. [Google Scholar] [CrossRef]
- Shew, B.; Kuo, C.; Huang, Y.; Tsai, Y. UV-LIGA interferometer biosensor based on the SU-8 optical waveguide. Sens. Actuators Phys. 2005, 120, 383–389. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X. Cellular mechanics study in cardiac myocytes using PDMS pillars array. Sens. Actuators Phys. 2006, 125, 398–404. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.-K.; Yang, M.T.; Desai, R.A.; Yu, X.; Liu, Z.; Chen, C.S. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods 2010, 7, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Hanada, K.; Maeda, R. A polydimethylsiloxane (PDMS) deformable diffraction grating for monitoring of local pressure in microfluidic devices. J. Micromech. Microeng. 2001, 12, 1. [Google Scholar] [CrossRef]
- Pishgar, R.; Wijdenes, P.; Iqbal, F.; Haidar, K.; Syeda, A.; Syed, N.; Dalton, C. Impact of open surface area of multi-well microelectrode array on mammalian brain cells recording efficiency. Int. Soc. Opt. Photonics 2020, 11235, 112350E. [Google Scholar]
- Shimba, K.; Sakai, K.; Iida, S.; Kotani, K.; Jimbo, Y. Long-term developmental process of the human cortex revealed in vitro by axon-targeted recording using a microtunnel-augmented microelectrode array. IEEE Trans. Biomed. Eng. 2019, 66, 2538–2545. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Wu, M.-D.; Shih, W.-P. A mask-free fabrication of SU-8/silicon spherical microprobe. Microelectron. Eng. 2011, 88, 2129–2131. [Google Scholar] [CrossRef]
- Altuna, A.; de la Prida, L.M.; Bellistri, E.; Gabriel, G.; Guimerá, A.; Berganzo, J.; Villa, R.; Fernández, L.J. SU-8 based microprobes with integrated planar electrodes for enhanced neural depth recording. Biosens. Bioelectron. 2012, 37, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Lee, J.-B. High aspect ratio tapered hollow metallic microneedle arrays with microfluidic interconnector. Microsyst. Technol. 2007, 13, 231–235. [Google Scholar] [CrossRef]
- Rogers, J.A.; Jackman, R.J.; Schueller, O.J.; Whitesides, G.M. Elastomeric diffraction gratings as photothermal detectors. Appl. Opt. 1996, 35, 6641–6647. [Google Scholar] [CrossRef]
- Gibbons, A.; Láng, O.; Kojima, Y.; Ito, M.; Ono, K.; Tanaka, K.; Sivaniah, E. Real-time visualization of cardiac cell beating behaviour on polymer diffraction gratings. RSC Adv. 2017, 7, 51121–51129. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Lee, S.-H.; Kim, Y.-K. A high-aspect-ratio comb actuator using UV-LIGA surface micromachining and (110) silicon bulk micromachining. J. Micromech. Microeng. 2002, 12, 128. [Google Scholar] [CrossRef]
- Bustillo, J.M.; Howe, R.T.; Muller, R.S. Surface micromachining for microelectromechanical systems. Proc. IEEE 1998, 86, 1552–1574. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, K.; Chen, K.; Xu, J.; Ma, Z.; Li, W.; Xu, L.; Huang, X. Fabrication and characterization of Si nanotip arrays for Si-based nano-devices. Int. Soc. Opt. Photonics 2008, 6984, 698426. [Google Scholar]
- Tsai, J.-C.; Hsu, Y.-S. Profile of microlens fabricated by the thermal reflow process. IEEE Trans. Magn. 2011, 47, 598–601. [Google Scholar] [CrossRef]
- Jiang, G.; Baig, S.; Wang, M.R. Prism-assisted inclined UV lithography for 3D microstructure fabrication. J. Micromech. Microeng. 2012, 22, 85022. [Google Scholar] [CrossRef]
- Taniguchi, J.; Koga, K.; Kogo, Y.; Miyamoto, I. Rapid and three-dimensional nanoimprint template fabrication technology using focused ion beam lithography. Microelectron. Eng. 2006, 83, 940–943. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Grob, L.; Yamamoto, H.; Zips, S.; Rinklin, P.; Hirano-Iwata, A.; Wolfrum, B. Printed 3D Electrode Arrays with Micrometer-Scale Lateral Resolution for Extracellular Recording of Action Potentials. Adv. Mater. Technol. 2020, 5, 1900517. [Google Scholar] [CrossRef]
- Hu, Y.; Lao, Z.; Cumming, B.P.; Wu, D.; Li, J.; Liang, H.; Chu, J.; Huang, W.; Gu, M. Laser printing hierarchical structures with the aid of controlled capillary-driven self-assembly. Proc. Natl. Acad. Sci. USA 2015, 112, 6876–6881. [Google Scholar] [CrossRef] [Green Version]
- Jafari, R.; Cloutier, C.; Allahdini, A.; Momen, G. Recent progress and challenges with 3D printing of patterned hydrophobic and superhydrophobic surfaces. Int. J. Adv. Manuf. Technol. 2019, 103, 1225–1238. [Google Scholar] [CrossRef]
- Cheng, M.; Deivanayagam, R.; Shahbazian-Yassar, R. 3D printing of electrochemical energy storage devices: A review of printing techniques and electrode/electrolyte architectures. Batter. Supercaps 2020, 3, 130–146. [Google Scholar] [CrossRef]
- Kim, K.; Park, D.S.; Lu, H.M.; Che, W.; Kim, K.; Lee, J.-B.; Ahn, C.H. A tapered hollow metallic microneedle array using backside exposure of SU-8. J. Micromech. Microeng. 2004, 14, 597. [Google Scholar] [CrossRef]
- Ghosh, S.; Ananthasuresh, G. Single-photon-multi-layer-interference lithography for high-aspect-ratio and three-dimensional SU-8 micro-/nanostructures. Sci. Rep. 2016, 6, 18428. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, H.; Ryu, H.-Y.; An, I.S.; Oh, H.-K.; Ahn, J.-H.; Park, J.-G. Dimensionally controlled complex 3D sub-micron pattern fabrication by single step dual diffuser lithography (DDL). Microelectron. Eng. 2015, 143, 25–30. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Weber, A.; Li, W. Varying-length polymer microneedle arrays fabricated by droplet backside exposure. J. Microelectromech. Syst. 2014, 23, 1272–1280. [Google Scholar] [CrossRef]
- SU-8 2000 Permanent Epoxy Negative Photoresist PROCESSING GUIDELINES FOR: SU-8 2100 and SU-8 2150, Kayakuam. Available online: https://kayakuam.com/wp-content/uploads/2019/09/SU-82000DataSheet2100and2150Ver5.pdf (accessed on 28 September 2020).
- Kang, W.-J.; Rabe, E.; Kopetz, S.; Neyer, A. Novel exposure methods based on reflection and refraction effects in the field of SU-8 lithography. J. Micromech. Microeng. 2006, 16, 821. [Google Scholar] [CrossRef]
- Xu, F.; Wu, C.M.; Rengarajan, V.; Finley, T.D.; Keles, H.O.; Sung, Y.; Li, B.; Gurkan, U.A.; Demirci, U. Three-dimensional magnetic assembly of microscale hydrogels. Adv. Mater. 2011, 23, 4254–4260. [Google Scholar] [CrossRef] [Green Version]
- Spaepen, P.; De Boodt, S.; Aerts, J.-M.; Vander Sloten, J. Digital image processing of live/dead staining. In Mammalian Cell Viability; Springer: Berlin/Heidelberg, Germany, 2011; pp. 209–230. [Google Scholar]
- Hart, S.R.; Huang, Y.; Fothergill, T.; Lumbard, D.C.; Dent, E.W.; Williams, J.C. Adhesive micro-line periodicity determines guidance of axonal outgrowth. Lab. Chip 2013, 13, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.; Zhang, X. Optically processed microlens array for single-beam lithography of plasmonic structures. Nanophotonics 2018, 7, 1819–1825. [Google Scholar] [CrossRef]
- De Boor, J.; Geyer, N.; Wittemann, J.V.; Gösele, U.; Schmidt, V. Sub-100 nm silicon nanowires by laser interference lithography and metal-assisted etching. Nanotechnology 2010, 21, 95302. [Google Scholar] [CrossRef] [Green Version]
- Quiñónez, F.; Menezes, J.; Cescato, L.; Rodriguez-Esquerre, V.; Hernandez-Figueroa, H.; Mansano, R. Band gap of hexagonal 2D photonic crystals with elliptical holes recorded by interference lithography. Opt. Express 2006, 14, 4873–4879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, M.E. On the Design of Lithographic Interferometers and Their Application. Ph.D. thesis, Massachusetts Institute of Technology, September 2004. [Google Scholar]
- Brueck, S.R. Optical and interferometric lithography-nanotechnology enablers. Proc. IEEE 2005, 93, 1704–1721. [Google Scholar] [CrossRef]
- Crozier, S.D., Jr. Development of Interference Lithography Capability Using a Helium Cadmium Ultraviolet Multimode Laser for the Fabrication of Sub-Micron-Structured Optical Materials; AFIT/GE/ENG/11-07; Air Force Inst of Tech Wright-Patterson AFB OH School of Engineering and Management: Wright-Patterson Air Force Base, OH, USA, 2011. [Google Scholar]
- Chen, X.; Zaidi, S.H.; Brueck, S.; Devine, D.J. Interferometric lithography of sub-micrometer sparse hole arrays for field-emission display applications. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1996, 14, 3339–3349. [Google Scholar] [CrossRef]
- Bozler, C.O.; Harris, C.T.; Rabe, S.; Rathman, D.D.; Hollis, M.A.; Smith, H.I. Arrays of gated field-emitter cones having 0.32 μm tip-to-tip spacing. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1994, 12, 629–632. [Google Scholar] [CrossRef]
- Dendukuri, D.; Panda, P.; Haghgooie, R.; Kim, J.M.; Hatton, T.A.; Doyle, P.S. Modeling of oxygen-inhibited free radical photopolymerization in a PDMS microfluidic device. Macromolecules 2008, 41, 8547–8556. [Google Scholar] [CrossRef] [Green Version]
- Alvankarian, J.; Majlis, B.Y. Exploiting the oxygen inhibitory effect on UV curing in microfabrication: A modified lithography technique. PLoS ONE 2015, 10, e0119658. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, D.J.; DiMenna, W.; Flaim, T.D.; Mercado, R.; Sun, S. Dyed red, green, and blue photoresist for manufacture of high-resolution color filter arrays for image sensors. Int. Soc. Opt. Photonics 2003, 5017, 298–306. [Google Scholar]
- Varapnickas, S.; Malinauskas, M. Processes of Direct Laser Writing 3D Nano-Lithography. Preprints 2018. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chan-Park, M.B.; Conner, S.R. Effect of exposure dose on the replication fidelity and profile of very high aspect ratio microchannels in SU-8. Lab. Chip 2004, 4, 646–653. [Google Scholar] [CrossRef]
- Li, H.; Fan, Y.; Conchouso, D.; Foulds, I.G. Surface tension-induced PDMS micro-pillars with controllable tips and tilt angles. Microsyst. Technol. 2015, 21, 445–449. [Google Scholar] [CrossRef]
- Sanjay, S.L.; Annaso, B.G.; Chavan, S.M.; Rajiv, S.V. Recent progress in preparation of superhydrophobic surfaces: A review. J. Surf. Eng. Mater. Adv. Technol. 2012, 2, 76–94. [Google Scholar]
- Law, K.-Y. Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: Getting the basics right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Miwa, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Effects of the surface roughness on sliding angles of water droplets on superhydrophobic surfaces. Langmuir 2000, 16, 5754–5760. [Google Scholar] [CrossRef]
- Li, X.-M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, P.; Mammen, L.; Deng, X.; Vollmer, D.; Butt, H.-J. How superhydrophobicity breaks down. Proc. Natl. Acad. Sci. USA 2013, 110, 3254–3258. [Google Scholar] [CrossRef] [Green Version]
- Cassie, A. Contact angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Li, F.; Hou, H.; Yin, J.; Jiang, X. Near-infrared light–responsive dynamic wrinkle patterns. Sci. Adv. 2018, 4, eaar5762. [Google Scholar] [CrossRef] [Green Version]
- Bajgrowicz-Cieslak, M.; Alqurashi, Y.; Elshereif, M.I.; Yetisen, A.K.; Hassan, M.U.; Butt, H. Optical glucose sensors based on hexagonally-packed 2.5-dimensional photonic concavities imprinted in phenylboronic acid functionalized hydrogel films. RSC Adv. 2017, 7, 53916–53924. [Google Scholar] [CrossRef] [Green Version]
- England, G.; Kolle, M.; Kim, P.; Khan, M.; Muñoz, P.; Mazur, E.; Aizenberg, J. Bioinspired micrograting arrays mimicking the reverse color diffraction elements evolved by the butterfly Pierella luna. Proc. Natl. Acad. Sci. USA 2014, 111, 15630–15634. [Google Scholar] [CrossRef] [Green Version]
- Min, K.-P.; Kim, J.; Song, K.D.; Kim, G.-W. A G-Fresnel Optical Device and Image Processing Based Miniature Spectrometer for Mechanoluminescence Sensor Applications. Sensors 2019, 19, 3528. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Gai, M.; Ignatov, A.V.; Dyakov, S.A.; Wang, J.; Gippius, N.A.; Frueh, J.; Sukhorukov, G.B. Stimuli-Responsive Microarray Films for Real-Time Sensing of Surrounding Media, Temperature, and Solution Properties via Diffraction Patterns. ACS Appl. Mater. Interfaces 2020, 12, 19080–19091. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Wang, K.; Akram, M.; Henriksen, L.; Chen, X. Optimization of PDMS network for a fast response and sensitive actuation material applied in a MEMS spatial light modulator. Appl. Phys. A 2011, 103, 381–388. [Google Scholar] [CrossRef]
- Ryabchun, A.; Wegener, M.; Gritsai, Y.; Sakhno, O. Novel effective approach for the fabrication of PDMS-based elastic volume gratings. Adv. Opt. Mater. 2016, 4, 169–176. [Google Scholar] [CrossRef]
- Suslik, L.; Pudis, D.; Goraus, M.; Nolte, R.; Kovac, J.; Durisova, J.; Gaso, P.; Hronec, P.; Schaaf, P. Photonic crystal and photonic quasicrystal patterned in PDMS surfaces and their effect on LED radiation properties. Appl. Surf. Sci. 2017, 395, 220–225. [Google Scholar] [CrossRef]
- Ghar, A.; Das, U.; Panigrahi, P. Higher order micro transmission grating fabrication inside quartz glass by femtosecond laser micromachining. In Proceedings of the 2017 IEEE Photonics Conference (IPC), Orlando, FL, USA, 1–5 October 2017; pp. 641–642. [Google Scholar]
- Le Saux, G.; Magenau, A.; Böcking, T.; Gaus, K.; Gooding, J.J. The relative importance of topography and RGD ligand density for endothelial cell adhesion. PLoS ONE 2011, 6, e21869. [Google Scholar] [CrossRef] [Green Version]
- Kolind, K.; Dolatshahi-Pirouz, A.; Lovmand, J.; Pedersen, F.S.; Foss, M.; Besenbacher, F. A combinatorial screening of human fibroblast responses on micro-structured surfaces. Biomaterials 2010, 31, 9182–9191. [Google Scholar] [CrossRef]
- Froeter, P.; Huang, Y.; Cangellaris, O.V.; Huang, W.; Dent, E.W.; Gillette, M.U.; Williams, J.C.; Li, X. Toward intelligent synthetic neural circuits: Directing and accelerating neuron cell growth by self-rolled-up silicon nitride microtube array. ACS Nano 2014, 8, 11108–11117. [Google Scholar] [CrossRef] [Green Version]
- Mrksich, M.; Whitesides, G.M. Patterning self-assembled monolayers using microcontact printing: A new technology for biosensors? Trends Biotechnol. 1995, 13, 228–235. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, C.; He, Q.; Chen, J.; Yu, D.; Li, J.; Zhai, S.; Qin, Z.; Du, K.; Chu, Z. Current and Perspective Diagnostic Techniques for COVID-19. ACS Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Hass, K.; Bao, M.; He, Q.; Park, M.; Qin, P.; Du, K. Integrated Micropillar Polydimethylsiloxane Accurate CRISPR Detection (IMPACT) System for Rapid Viral DNA Sensing. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Oliva, A.A.; James, C.D.; Kingman, C.E.; Craighead, H.G.; Banker, G.A. Patterning axonal guidance molecules using a novel strategy for microcontact printing. Neurochem. Res. 2003, 28, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Khadpekar, A.J.; Khan, M.; Sose, A.; Majumder, A. Low Cost and Lithography-free stamp fabrication for Microcontact printing. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Von Philipsborn, A.C.; Lang, S.; Bernard, A.; Loeschinger, J.; David, C.; Lehnert, D.; Bastmeyer, M.; Bonhoeffer, F. Microcontact printing of axon guidance molecules for generation of graded patterns. Nat. Protoc. 2006, 1, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Renault, J.P.; Michel, B.; Bosshard, H.R.; Delamarche, E. Microcontact printing of proteins. Adv. Mater. 2000, 12, 1067–1070. [Google Scholar] [CrossRef]
- Schwaab, D.; Zentis, P.; Winter, S.; Meffert, S.; Offenhäusser, A.; Mayer, D. Generation of protein nanogradients by microcontact printing. Jpn. J. Appl. Phys. 2013, 52, 5DA19. [Google Scholar] [CrossRef]







| Sample | Change in Parameter | First Exposure Energy (mJ/cm2) | Wait Time between the Exposures (s) | The Angle of Rotation (°) | Second Exposure (mJ/cm2) |
|---|---|---|---|---|---|
| Dome | Initial parameters | 350 | 0 | 90 | 350 |
| Petal | Half the angle of rotation | 350 | 0 | 45 | 350 |
| Bell | Half the exposures | 175 | 0 | 90 | 175 |
| Bullet | Wait time between exposures | 350 | 60 | 90 | 350 |
| Pillar | Half the exposures and wait time (From Bullet) | 175 | 30 | 90 | 175 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rengarajan, V.; Geng, J.; Huang, Y. Fabrication of Tapered 3D Microstructure Arrays Using Dual-Exposure Lithography (DEL). Micromachines 2020, 11, 903. https://doi.org/10.3390/mi11100903
Rengarajan V, Geng J, Huang Y. Fabrication of Tapered 3D Microstructure Arrays Using Dual-Exposure Lithography (DEL). Micromachines. 2020; 11(10):903. https://doi.org/10.3390/mi11100903
Chicago/Turabian StyleRengarajan, Venkatakrishnan, Junnan Geng, and Yu Huang. 2020. "Fabrication of Tapered 3D Microstructure Arrays Using Dual-Exposure Lithography (DEL)" Micromachines 11, no. 10: 903. https://doi.org/10.3390/mi11100903
APA StyleRengarajan, V., Geng, J., & Huang, Y. (2020). Fabrication of Tapered 3D Microstructure Arrays Using Dual-Exposure Lithography (DEL). Micromachines, 11(10), 903. https://doi.org/10.3390/mi11100903