Hydrogel Patterns in Microfluidic Devices by Do-It-Yourself UV-Photolithography Suitable for Very Large-Scale Integration
Abstract
:1. Introduction
2. Materials and Methods
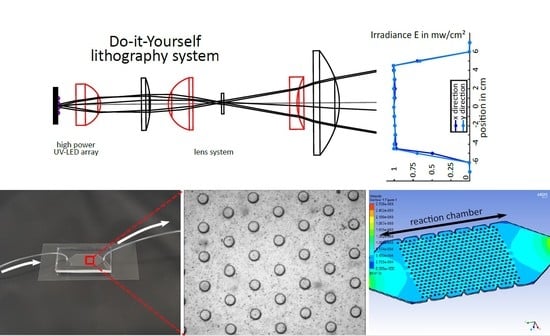
2.1. Photolithographic Set-Up
2.2. Preparation of the Hydrogel Precursor
2.3. Photopolymerization Process
2.4. Manufacturing of the Microfluidic Systems and Hydrogel Integration
2.4.1. Flip-Chip Method
2.4.2. In Situ Polymerization
2.5. Preperation of Hydrogel-Based Enzymatic Microreactors
3. Results
3.1. Photopolymerization of Hydrogel Patterns and Characterization
3.2. Comparison of the Methods for Hydrogel High-Density Integration
3.3. Hydrogel-Based Microreactors: Increasing Yield by Miniaturization
3.3.1. Design of the Microfluidic Device
3.3.2. Enzymatic Conversion in Dependence of SA:V Ratio: Optimization and Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Fine-Tuning the UV Exposure System
Appendix B
Optimizations for the Polymerization Process

Appendix C
Challenges Due to Miniaturization: The Leaching Effect

References
- Neil, C.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorsen, T.; Maerkl, S.J.; Quake, S.R. Microfluidic large-scale integration. Science 2002, 298, 580–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araci, I.E.; Quake, S.R. Microfluidic very large scale integration (mVLSI) with integrated micromechanical valves. Lab Chip 2012, 12, 2803–2806. [Google Scholar] [CrossRef] [PubMed]
- Melin, J.; Lee, A.; Foygel, K.; Leong, D.E.; Quake, S.R.; Yao, M.W. In vitro embryo culture in DEfiNED, sub-microliter volumes. Dev. Dyn. 2009, 238, 950–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadwen, B.; Broder, G.R.; Morganti, D.; Jacobs, A.; Brown, C.; Hector, J.R.; Kubota, Y.; Morgan, H. Programmable large area digital microfluidic array with integrated droplet sensing for bioassays. Lab Chip 2012, 12, 3305–3313. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Kuckling, D.; Howitz, S.; Gehring, T.; Arndt, K.-F. Electronically controllable microvalves based on smart hydrogels: Magnitudes and potential applications. J. Microelectromech. Syst. 2003, 12, 748–753. [Google Scholar] [CrossRef]
- Ehrenhofer, A.; Bingel, G.; Paschew, G.; Tietze, M.; Schroeder, R.; Richter, A.; Wallmersperger, T. Permeation control in hydrogel-layered patterned PET membranes with defined switchable pore geometry—Experiments and numerical simulation. Sens. Actuators B Chem. 2016, 232, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Richter, A.; Klatt, S.; Paschew, G.; Christian, K. Micropumps operated by swelling and shrinking of temperature-sensitive hydrogels. Lab Chip 2009, 9, 613–618. [Google Scholar] [CrossRef]
- Richter, A.; Tuerke, A.; Andrij, P. Controlled double-sensitivity of microgels applied to electronically adjustable chemostats. Adv. Mater. 2007, 19, 1109–1112. [Google Scholar] [CrossRef]
- Haefner, S.; Frank, P.; Elstner, M.; Nowak, J.; Odenbach, S.; Richter, A. Smart hydrogels as storage elements with dispensing functionality in discontinuous microfluidic systems. Lab Chip 2016, 16, 3977–3989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haefner, S.; Frank, P.; Langer, E.; Gruner, D.; Schmidt, U.; Elsnter, M.; Gerlach, G.; Richter, A. Chemically controlled micro-pores and nano-filters for separation tasks in 2D and 3D microfluidic systems. RSC Adv. 2017, 7, 49279–49289. [Google Scholar] [CrossRef] [Green Version]
- Simon, D.; Obst, F.; Haefner, S.; Heroldt, T.; Peiter, M.; Simon, F.; Richter, A.; Voit, B.; Appelhans, D. Hydrogel/enzyme dots as adaptable tool for non-compartmentalized multi-enzymatic reactions in microfluidic devices. React. Chem. Eng. 2019, 4, 67–77. [Google Scholar] [CrossRef]
- Obst, F.; Simon, D.; Mehner, P.J.; Neubauer, J.W.; Beck, A.; Stroyuk, O.; Richter, A.; Voit, B.; Appelhans, D. One-step photostructuring of multiple hydrogel arrays for compartmentalized enzyme reactions in microfluidic devices. React. Chem. Eng. 2019, 4, 2141–2155. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G. Optoelectrothermic control of highly integrated polymer-based MEMS applied in an artificial skin. Adv. Mater. 2009, 21, 979–983. [Google Scholar] [CrossRef]
- Greiner, R.; Allerdissen, M.; Voigt, A.; Richter, A. Fluidic microchemomechanical integrated circuits processing chemical information. Lab Chip 2012, 12, 5034–5044. [Google Scholar] [CrossRef] [Green Version]
- Frank, P.; Schreiter, J.; Haefner, S.; Paschew, G.; Voigt, A.; Richter, A. Integrated microfluidic membrane transistor utilizing chemical information for on-chip flow control. PLoS ONE 2016, 11, e0161024. [Google Scholar] [CrossRef] [Green Version]
- Paschew, G.; Schreiter, J.; Voigt, A.; Pini, C.; Chávez, J.P.; Allerdissen, M.; Marschner, U.; Siegmund, S.; Juelicher, F.; Schueffny, R.; et al. Autonomous chemical oscillator circuit based on bidirectional chemical-microfluidic coupling. Adv. Mater. Technol. 2016, 1, 1600005. [Google Scholar] [CrossRef] [Green Version]
- Frank, P.; Gräfe, D.; Probst, C.; Haefner, S.; Elstner, M.; Appelhans, D.; Kohlheyer, D.; Voit, B.; Richter, A. Autonomous integrated microfluidic circuits for chip-level flow control utilizing chemofluidic transistors. Adv. Funct. Mater. 2017, 27, 1700430. [Google Scholar] [CrossRef]
- Gerald, G.; Arndt, K.-F. Hydrogel Sensors and Actuators: Engineering and Technology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 6, pp. 15–68. [Google Scholar]
- Gerlach, G.; Guenther, M.; Sorber, J.; Suchaneck, G.; Arndt, K.-F.; Richter, A. Chemical and pH sensors based on the swelling behavior of hydrogels. Sens. Actuators B Chem. 2005, 111, 555–561. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Haswell, S.; Gibson, I. Lab-on-a-chip or chip-in-a-lab: Challenges of commercialization lost in translation. Procedia Technol. 2015, 20, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Chandra, D.; Taylor, J.A.; Yang, S. Replica molding of high-aspect-ratio (sub-)micron hydrogel pillar arrays and their stability in air and solvents. Soft. Matter. 2008, 4, 979–984. [Google Scholar] [CrossRef]
- Chollet, B.; Li, M.; Martwong, E.; Bresson, B.; Fretigny, C.; Tabeling, P.; Tran, Y. Multiscale surface-attached hydrogel thin films with tailored architecture. ACS Appl. Mater. Interfaces 2016, 8, 11729–11738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagawa, F.; Sugiura, S.; Kanamori, T. Hydrogel microfabrication technology toward three dimensional tissue engineering. Regen. Ther. 2016, 3, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Ploetner, M.; Kuckling, D.; Fischer, W.-J. Photopatterning of thermally sensitive hydrogels useful for microactuators. Sens. Actuators A Phys. 1999, 77, 139–144. [Google Scholar] [CrossRef]
- Kuckling, D.; Adler, H.-J.P.; Arndt, K.-F.; Hoffmann, J.; Ploetner, M.; Wolff, T. Photocrosslinking of thin polymer film of temperature sensitive polymers. Polym. Adv. Technol. 1999, 10, 345–352. [Google Scholar] [CrossRef]
- D’Eramo, L.; Chollet, B.; Leman, M.; Martwong, E.; Li, M.; Geisler, H.; Dupire, J.; Kerdraon, M.; Vergne, C.; Monti, F.; et al. Microfluidic actuators based on temperature-responsive hydrogels. Microsyst. Nanoeng. 2018, 4, 17069. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.; Ko, S.Y.; Park, J.-O.; Akella, S.; Xu, B.; Zhang, Y.; Fraden, S. Controlling uniformity of photopolymerized microscopic hydrogels. Lab Chip 2014, 14, 1551–1563. [Google Scholar] [CrossRef] [Green Version]
- Richter, A.; Wenzel, J.; Kretschmer, K. Mechanically adjustable chemostats based on stimuli-responsive polymers. Sens. Actuators B Chem. 2007, 125, 569–573. [Google Scholar] [CrossRef]
- Harder, I.; Lano, M.; Lindlein, N.; Schwider, J. Homogenization and beam shaping with microlens arrays. Photon. Manag. 2004, 5456, 99–108. [Google Scholar]
- Jin, Y.; Hassan, A.; Jiang, Y. Freeform microlens array homogenizer for excimer laser beam shaping. Opt. Express 2016, 24, 24846–24858. [Google Scholar] [CrossRef] [PubMed]
- Haefner, S.; Rohn, M.; Frank, P.; Paschew, G.; Elstner, M.; Richter, A. Improved PNIPAAm-hydrogel photopatterning by process optimisation with respect to UV light sources and oxygen content. Gels 2016, 2, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, S.S. Shrinkage ratio of PDMS and its alignment method for the wafer level process. Microsyst. Technol. 2008, 14, 205–208. [Google Scholar] [CrossRef]
- Haefner, S.; Koerbitz, R.; Frank, P.; Elstner, M.; Richter, A. High integration of microfluidic circuits based on hydrogel valves for MEMS control. Adv. Mater. Technol. 2018, 3, 1700108. [Google Scholar] [CrossRef]
- Beebe, D.J.; Moore, J.S.; Bauer, J.M.; Yu, Q.; Liu, R.H.; Devadoss, C.; Jo, B.-H. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature 2000, 404, 588. [Google Scholar] [CrossRef]
- Yu, T.-W.; Ong, C.N. Lag-time measurement of antioxidant capacity using myoglobin and 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid): Rationale, application, and limitation. Anal. Biochem. 1999, 275, 217–223. [Google Scholar] [CrossRef]
- Moraes, C.; Sun, Y.; Simmons, C.A. Solving the shrinkage-induced PDMS alignment registration issue in multilayer soft lithography. J. Micromech. Microeng. 2009, 19, 065015. [Google Scholar] [CrossRef]
- Zhan, W.; Seong, G.H.; Crooks, R.M. Hydrogel-based microreactors as a functional component of microfluidic systems. Anal. Chem. 2002, 74, 4647–4652. [Google Scholar] [CrossRef]
- Mehner, P.J.; Obst, F.; Simon, D.; Tang, J.; Beck, A.; Gruner, D.; Busek, M.; Appelhans, D.; Marschner, U.; Voit, B.; et al. Modeling hydrogel-controlled micro-reactors for enzyme assays with finite elements for improved flow and filling distribution. In Proceedings of the ASME 2018 Conference on Smart Materials, Adaptive Structures and Intelligent Systems, San Antonio, TX, USA, 10–12 September 2018. [Google Scholar]
- Zhang, X.; Li, L.; Luo, C. Gel integration for microfluidic applications. Lab Chip 2016, 16, 1757–1776. [Google Scholar] [CrossRef] [PubMed]
- Demello, A.J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Crooks, R.M. Microfluidic biosensor based on an array of hydrogel-entrapped enzymes. Anal. Chem. 2005, 77, 6843–6851. [Google Scholar] [CrossRef] [PubMed]
- Logan, T.C.; Clark, D.S.; Stachowiak, T.B.; Svec, F.; Frechet, J.M.J. Photopatterning enzymes on polymer monoliths in microfluidic devices for steady-state kinetic analysis and spatially separated multi-enzyme reactions. Anal. Chem. 2007, 79, 6592–6598. [Google Scholar] [CrossRef]
- Odom, T.; Huntington, M. Benchtop photolithography tool offers a low-cost route to nanomanufacturing. Int. Soc. Opt. Photon. SPIE 2012. [Google Scholar] [CrossRef] [Green Version]
- Luminus Devices Inc. Mosaic Array Series—Ultraviolet Chip on Board LEDs. Available online: https://download.luminus.com/datasheets/Luminus_CBM-120-UV-Gen3_Datasheet.pdf (accessed on 12 December 2019).
- Kangning, R.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in microfluidic materials, functions, integration, and applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhu, M.; Liu, X.; Zhang, W.; Sun, B.; Chen, Y.; Adler, H.-J.P. High clay content nanocomposite hydrogels with surprising mechanical strength and interesting deswelling kinetics. Polymer 2006, 47, 1–5. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef] [Green Version]









| Reactor | Pillar Diameter (µm) | Reactor Height (µm) | Number of Pillars | Total Hydrogel Volume (µL) | Total SA of Hydrogel (mm²) | SA:V Increase Relative to R1 |
|---|---|---|---|---|---|---|
| R1 | 350 | 150 | 104 | 1,51 | 17.3 | 1.0 |
| R2 | 150 | 150 | 570 | 1,51 | 40.3 | 2,3 |
| R3 | 50 | 150 | 5132 | 1,51 | 120.9 | 7.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, A.; Obst, F.; Busek, M.; Grünzner, S.; Mehner, P.J.; Paschew, G.; Appelhans, D.; Voit, B.; Richter, A. Hydrogel Patterns in Microfluidic Devices by Do-It-Yourself UV-Photolithography Suitable for Very Large-Scale Integration. Micromachines 2020, 11, 479. https://doi.org/10.3390/mi11050479
Beck A, Obst F, Busek M, Grünzner S, Mehner PJ, Paschew G, Appelhans D, Voit B, Richter A. Hydrogel Patterns in Microfluidic Devices by Do-It-Yourself UV-Photolithography Suitable for Very Large-Scale Integration. Micromachines. 2020; 11(5):479. https://doi.org/10.3390/mi11050479
Chicago/Turabian StyleBeck, Anthony, Franziska Obst, Mathias Busek, Stefan Grünzner, Philipp J. Mehner, Georgi Paschew, Dietmar Appelhans, Brigitte Voit, and Andreas Richter. 2020. "Hydrogel Patterns in Microfluidic Devices by Do-It-Yourself UV-Photolithography Suitable for Very Large-Scale Integration" Micromachines 11, no. 5: 479. https://doi.org/10.3390/mi11050479
APA StyleBeck, A., Obst, F., Busek, M., Grünzner, S., Mehner, P. J., Paschew, G., Appelhans, D., Voit, B., & Richter, A. (2020). Hydrogel Patterns in Microfluidic Devices by Do-It-Yourself UV-Photolithography Suitable for Very Large-Scale Integration. Micromachines, 11(5), 479. https://doi.org/10.3390/mi11050479