Monolithically Integrated Diffused Silicon Two-Zone Heaters for Silicon-Pyrex Glass Microreactors for Production of Nanoparticles: Heat Exchange Aspects
Abstract
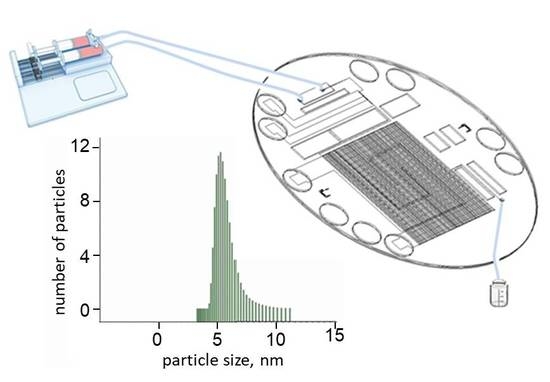
:1. Introduction
2. Fabrication
3. Results and Discussion
3.1. Simulation
3.2. Experimental Results
4. Conclusions and Future Works for Verification of the Design and Results
Author Contributions
Funding
Conflicts of Interest
References
- Pohar, A.; Lakner, M.; Plazl, I. Parallel flow of immiscible liquids in a microreactor: Modeling and experimental study. Microfluid. Nanofluidics 2012, 12, 307–316. [Google Scholar] [CrossRef]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Lagally, E.T.; Mathies, R.A. Integrated genetic analysis microsystems. J. Phys. D Appl. Phys. 2004, 37, R245–R261. [Google Scholar] [CrossRef]
- Nielsen, C.A.; Chrisman, R.W.; LaPointe, R.E.; Miller, T.E. Novel tubing microreactor for monitoring chemical reactions. Anal. Chem. 2002, 74, 3112–3117. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, S.H. Micro reactors for chemical synthesis. Curr. Opin. Chem. Biol. 1999, 3, 350–356. [Google Scholar] [CrossRef]
- Jia, H.; Wong, Y.L.; Jian, A.; Tsoi, C.C.; Wang, M.; Li, W.; Zhang, W.; Sang, S.; Zhang, X. Microfluidic Reactors for Plasmonic Photocatalysis Using Gold Nanoparticles. Micromachines 2019, 10, 869. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, J.I.; Nagaki, A.; Yamada, T. Flash chemistry: Fast chemical synthesis by using microreactors. Chem. Eur. J. 2008, 14, 7450–7459. [Google Scholar] [CrossRef]
- Zhao, C.-X.; He, L.; Qiao, S.Z.; Middelberg, A.P. Nanoparticle synthesis in microreactors. Chem. Eng. Sci. 2011, 66, 1463–1479. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Recent advances in micro reaction technology. Chem. Commun. 2011, 47, 6512–6535. [Google Scholar] [CrossRef]
- Yue, J.; Falke, F.H.; Schouten, J.C.; Nijhuis, T.A. Microreactors with integrated UV/Vis spectroscopic detection for online process analysis under segmented flow. Lab Chip 2013, 13, 4855–4863. [Google Scholar] [CrossRef] [Green Version]
- Ehgartner, J.; Sulzer, P.; Burger, T.; Kasjanow, A.; Bouwes, D.; Krühne, U.; Klimant, I.; Mayr, T. Online analysis of oxygen inside silicon-glass microreactors with integrated optical sensors. Sens. Actuators B Chem. 2016, 228, 748–757. [Google Scholar] [CrossRef] [Green Version]
- Mensinger, H.; Richter, T.; Hessel, V.; Döpper, J.; Ehrfeld, W. Microreactor with Integrated Static Mixer and Analysis System. In Micro Total Analysis Systems; Springer: Berlin/Heidelberg, Germany, 1995; pp. 237–243. [Google Scholar] [CrossRef]
- Pfeiffer, S.A.; Borisov, S.M.; Nagl, S. In-line monitoring of pH and oxygen during enzymatic reactions in off-the-shelf all-glass microreactors using integrated luminescent microsensors. Microchim. Acta 2017, 184, 621–626. [Google Scholar] [CrossRef]
- Abgrall, P.; Gue, A. Lab-on-chip technologies: Making a microfluidic network and coupling it into a complete microsystem—A review. J. Micromech. Microeng. 2007, 17, R15. [Google Scholar] [CrossRef]
- Reyes, D.R.; Iossifidis, D.; Auroux, P.-A.; Manz, A. Micro total analysis systems. 1. Introduction, theory, and technology. Anal. Chem. 2002, 74, 2623–2636. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Dhingra, A.; Im, H.; Gulari, E. A scalable silicon microreactor for preferential CO oxidation: Performance comparison with a tubular packed-bed microreactor. Appl. Catal. A General 2004, 274, 285–293. [Google Scholar] [CrossRef]
- De La Iglesia, O.; Sebastián, V.; Mallada, R.; Nikolaidis, G.; Coronas, J.; Kolb, G.; Zapf, R.; Hessel, V.; Santamaría, J. Preparation of Pt/ZSM-5 films on stainless steel microreactors. Catal. Today 2007, 125, 2–10. [Google Scholar] [CrossRef]
- Knitter, R.; Göhring, D.; Risthaus, P.; Hausselt, J. Microfabrication of ceramic microreactors. Microsyst. Technol. 2001, 7, 85–90. [Google Scholar] [CrossRef]
- Rašljić, M.; Građanski, I.; Smiljanić, M.M.; Janković, N.Z.; Lazić, Ž.; Cvetanović Zobenica, K. Microfabrication of Bifurcated Microchannels with PDMS and ABS. In Proceedings of the 4th International Conference on Electrical, Electronics and Computing Engineering, Phnom Penh, Cambodia, 14–16 June 2017; pp. 5–8, ISBN 978-86-7466-692-0. [Google Scholar]
- Yousuff, C.M.; Danish, M.; Ho, E.T.W.; Kamal Basha, I.H.; Hamid, N.H.B. Study on the optimum cutting parameters of an aluminum mold for effective bonding strength of a PDMS microfluidic device. Micromachines 2017, 8, 258. [Google Scholar] [CrossRef]
- Marre, S.; Adamo, A.; Basak, S.; Aymonier, C.; Jensen, K.F. Design and Packaging of Microreactors for High Pressure and High Temperature Applications. Ind. Eng. Chem. Res. 2010, 49, 11310–11320. [Google Scholar] [CrossRef]
- Kusakabe, K.; Miyagawa, D.; Gu, Y.; Maeda, H.; Morooka, S. Development of self-heating microreactor for catalytic reactions. J. Chem. Eng. Jpn. 2001, 34, 441–443. [Google Scholar] [CrossRef]
- Ari, J.; Louvet, G.; Ledemi, Y.; Célarié, F.; Morais, S.; Bureau, B.; Marre, S.; Nazabal, V.; Messaddeq, Y. Anodic bonding of mid-infrared transparent germanate glasses for high pressure-high temperature microfluidic applications. Sci. Technol. Adv. Mater. 2020, 21, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanks, H.; Maycock, P.; Sidles, P.; Danielson, G. Thermal conductivity of silicon from 300 to 1400 K. Phys. Rev. 1963, 130, 1743. [Google Scholar] [CrossRef]
- Ibach, H. Thermal expansion of silicon and zinc oxide (I). Phys. Status Solidi 1969, 31, 625–634. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Rauch, C.B.; Stevens, R.L.; Liu, R.H.; Lenigk, R.; Grodzinski, P. High sensitivity PCR assay in plastic micro reactors. Lab Chip 2002, 2, 179–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdem, E.Y.; Cheng, J.C.; Doyle, F.M.; Pisano, A.P. Multi-Temperature Zone, Droplet-based Microreactor for Increased Temperature Control in Nanoparticle Synthesis. Small 2014, 10, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, S.; Wang, Y.; Wang, T.; Luo, G. Continuous and ultrafast preparation of In(OH)3, InOOH, and In2O3 series in a microreactor for gas sensors. Industr. Eng. Chem. Res. 2019, 58, 2206–2216. [Google Scholar] [CrossRef]
- Wagner, J.; Köhler, J. Continuous synthesis of gold nanoparticles in a microreactor. Nano Lett. 2005, 5, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.J.; AL–Anbari, R.H.; Kadhim, G.R.; Salame, C.T. Exploring potential environmental applications of TiO2 nanoparticles. Energy Procedia 2017, 119, 332–345. [Google Scholar] [CrossRef]
- Paz, Y. Application of TiO2 photocatalysis for air treatment: Patents’ overview. Appl. Catal. B Environ. 2010, 99, 448–460. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef]
- Abdeslam, A.; Fouad, K.; Khalifa, A. Design and optimization of platinium heaters for gas sensor applications. Dig. J. Nanomater. Biostruct. 2020, 15, 133–141. [Google Scholar]
- Liu, Q.; Ding, G.; Wang, Y.; Yao, J. Thermal performance of micro hotplates with novel shapes based on single-layer SiO2 suspended film. Micromachines 2018, 9, 514. [Google Scholar] [CrossRef] [Green Version]
- Quiram, D.J.; Jensen, K.F.; Schmidt, M.A.; Mills, P.L.; Ryley, J.F.; Wetzel, M.D.; Kraus, D.J. Integrated microreactor system for gas-phase reactions. In Micro Instrumentation for High Throughput Experimentation and Process Intensification—A Tool for PAT; Wiley Online Library: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Tommasi, A.; Cocuzza, M.; Perrone, D.; Pirri, C.F.; Mosca, R.; Villani, M.; Delmonte, N.; Zappettini, A.; Calestani, D.; Marasso, S.L. Modeling, fabrication and testing of a customizable micromachined hotplate for sensor applications. Sensors 2017, 17, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resnik, D.; Vrtačnik, D.; Možek, M.; Pečar, B.; Amon, S. Experimental study of heat-treated thin film Ti/Pt heater and temperature sensor properties on a Si microfluidic platform. J. Micromech. Microeng. 2011, 21, 025025. [Google Scholar] [CrossRef]
- Rašljić, M.; Smiljanić, M.M.; Lazić, Ž.; Radulović, K.; Zobenica, K.C.; Radović, D.V. Two types of integrated heaters for synthesis of TiO2 nanoparticles in microreactors. In Proceedings of the 5th International Conference on Electrical, Electronic and Computing Engineering, Malang, Indonesia, 16–18 October 2018; pp. 11–14, ISBN 978 86 7466 752-1. [Google Scholar]
- Chan, E.M.; Mathies, R.A.; Alivisatos, A.P. Size-controlled growth of CdSe nanocrystals in microfluidic reactors. Nano Lett. 2003, 3, 199–201. [Google Scholar] [CrossRef]
- Yen, B.K.; Günther, A.; Schmidt, M.A.; Jensen, K.F.; Bawendi, M.G. A microfabricated gas–liquid segmented flow reactor for high-temperature synthesis: The case of CdSe quantum dots. Angew. Chem. 2005, 117, 5583–5587. [Google Scholar] [CrossRef]
- Winterton, J.D.; Myers, D.R.; Lippmann, J.M.; Pisano, A.P.; Doyle, F.M. A novel continuous microfluidic reactor design for the controlled production of high-quality semiconductor nanocrystals. J. Nanopart. Res. 2008, 10, 893–905. [Google Scholar] [CrossRef]
- Bayt, R.L.; Breuer, K.S. A silicon heat exchanger with integrated intrinsic-point heater demonstrated in a micropropulsion application. Sens. Actuators 2001, 249–255. [Google Scholar] [CrossRef]
- Tiggelaar, R.M.; Van Male, P.; Berenschot, J.; Gardeniers, J.; Oosterbroek, R.; De Croon, M.; Schouten, J.; van den Berg, A.; Elwenspoek, M.C. Fabrication of a high-temperature microreactor with integrated heater and sensor patterns on an ultrathin silicon membrane. Sens. Actuators A Phys. 2005, 119, 196–205. [Google Scholar] [CrossRef]
- Creemer, J.F.; Helveg, S.; Kooyman, P.J.; Molenbroek, A.M.; Zandbergen, H.W.; Sarro, P.M. A MEMS reactor for atomic-scale microscopy of nanomaterials under industrially relevant conditions. J. Microelectromech. Syst. 2010, 19, 254–264. [Google Scholar] [CrossRef]
- Nightingale, A.M.; de Mello, J.C. Microscale synthesis of quantum dots. J. Mater. Chem. 2010, 20, 8454–8463. [Google Scholar] [CrossRef]
- Malecha, K.; Pijanowska, D.G.; Golonka, L.J.; Torbicz, W. LTCC microreactor for urea determination in biological fluids. Sens. Actuators B Chem. 2009, 141, 301–308. [Google Scholar] [CrossRef]
- Martínez-Cisneros, C.S.; Gómez-de Pedro, S.; Puyol, M.; García-García, J.; Alonso-Chamarro, J. Design, fabrication and characterization of microreactors for high temperature syntheses. Chem. Eng. J. 2012, 211, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Mihailović, M.; Creemer, J.; Sarro, P. Monocrystalline Si-based microhotplate heater. In Proceedings of the SAFE/STW, Veldhoven, The Netherlands, 29–30 November 2007; pp. 608–611, ISBN 978-90-73461-49-9. [Google Scholar]
- Beuvier, T.; Panduro, E.A.C.; Kwaśniewski, P.; Marre, S.; Lecoutre, C.; Garrabos, Y.; Aymonier, C.; Calvignac, B.; Gibaud, A. Implementation of in situ SAXS/WAXS characterization into silicon/glass microreactors. Lab Chip 2015, 15, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, G.; Lichtenberg, H.; Pesek, J.; Sheppard, T.L.; Wang, W.; Schöttner, L.; Rinke, G.; Dittmeyer, R.; Grunwaldt, J.D. Continuous microfluidic synthesis of colloidal ultrasmall gold nanoparticles: In situ study of the early reaction stages and application for catalysis. React. Chem. Eng. 2017, 2, 876–884. [Google Scholar] [CrossRef]
- Cao, E.; Brett, G.; Miedziak, P.J.; Douthwaite, J.M.; Barrass, S.; McMillan, P.F.; Hutchings, G.J.; Gavriilidis, A. A micropacked-bed multi-reactor system with in situ raman analysis for catalyst evaluation. Catal. Today 2017, 283, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Solsona, M.; Vollenbroek, J.; Tregouet, C.; Nieuwelink, A.-E.; Olthuis, W.; Van Den Berg, A.; Weckhuysen, B.; Odijk, M. Microfluidics and catalyst particles. Lab Chip 2019, 19, 3575–3601. [Google Scholar] [CrossRef] [Green Version]
- Bojang, A.A.; Wu, H.-S. Design, Fundamental Principles of Fabrication, and Applications of Microreactors. Processes 2020, 8, 891. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Gumfekar, S.P.; Bhanvase, B.A.; Sonawane, S.H.; Pimplapure, M.S. A review on microreactors: Reactor fabrication, design, and cutting-edge applications. Chem. Eng. Sci. 2018, 189, 431–448. [Google Scholar] [CrossRef]
- Yue, J. Multiphase flow processing in microreactors combined with heterogeneous catalysis for efficient and sustainable chemical synthesis. Catal. Today 2018, 308, 3–19. [Google Scholar] [CrossRef]
- Ohishi, Y.; Xie, J.; Miyazaki, Y.; Aikebaier, Y.; Muta, H.; Kurosaki, K.; Yamanaka, S.; Uchida, N.; Tada, T. Thermoelectric properties of heavily boron-and phosphorus-doped silicon. Jpn. J. Appl. Phys. 2015, 54, 071301. [Google Scholar] [CrossRef]







| Material | Thermal Conductivity [W/mK] | Heat Capacity at Constant Pressure [J/kgK] | Electrical Conductivity [S/m] |
|---|---|---|---|
| Pure silicon | 131 | 700 | Approx. 0 |
| p+ silicon (heaters) | 75 | 750 | 120,000 |
| Gold | 317 | 129 | 45.6 × 106 |
| Silica | 1.4 | 730 | Approx. 0 |
| Variable | IH1 (mA) | IH2 (mA) | IH1 (mA) | IH2 (mA) | IH1 (mA) | IH2 (mA) | IH1 (mA) | IH2 (mA) |
|---|---|---|---|---|---|---|---|---|
| Current (mA) | 90 | 80 | 120 | 80 | 140 | 80 | 150 | 80 |
| Temperatures (°C) | 68.5 | 93 | 86 | 103 | 104 | 113 | 114.3 | 119.6 |
| Variable | IH1 (mA) | IH2 (mA) | IH1 (mA) | IH2 (mA) | IH1 (mA) | IH2 (mA) | IH1 (mA) | IH2 (mA) |
|---|---|---|---|---|---|---|---|---|
| Current (mA) | 90 | 80 | 120 | 80 | 140 | 80 | 150 | 80 |
| Temperatures (°C) | 64.8 | 89.8 | 86.6 | 101.2 | 104.8 | 110.7 | 116.3 | 116.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rašljić Rafajilović, M.; Radulović, K.; Smiljanić, M.M.; Lazić, Ž.; Jakšić, Z.; Stanisavljev, D.; Radović, D.V. Monolithically Integrated Diffused Silicon Two-Zone Heaters for Silicon-Pyrex Glass Microreactors for Production of Nanoparticles: Heat Exchange Aspects. Micromachines 2020, 11, 818. https://doi.org/10.3390/mi11090818
Rašljić Rafajilović M, Radulović K, Smiljanić MM, Lazić Ž, Jakšić Z, Stanisavljev D, Radović DV. Monolithically Integrated Diffused Silicon Two-Zone Heaters for Silicon-Pyrex Glass Microreactors for Production of Nanoparticles: Heat Exchange Aspects. Micromachines. 2020; 11(9):818. https://doi.org/10.3390/mi11090818
Chicago/Turabian StyleRašljić Rafajilović, Milena, Katarina Radulović, Milče M. Smiljanić, Žarko Lazić, Zoran Jakšić, Dragomir Stanisavljev, and Dana Vasiljević Radović. 2020. "Monolithically Integrated Diffused Silicon Two-Zone Heaters for Silicon-Pyrex Glass Microreactors for Production of Nanoparticles: Heat Exchange Aspects" Micromachines 11, no. 9: 818. https://doi.org/10.3390/mi11090818
APA StyleRašljić Rafajilović, M., Radulović, K., Smiljanić, M. M., Lazić, Ž., Jakšić, Z., Stanisavljev, D., & Radović, D. V. (2020). Monolithically Integrated Diffused Silicon Two-Zone Heaters for Silicon-Pyrex Glass Microreactors for Production of Nanoparticles: Heat Exchange Aspects. Micromachines, 11(9), 818. https://doi.org/10.3390/mi11090818