A Microfluidic Device for Automated High Throughput Detection of Ice Nucleation of Snomax®
Abstract
:1. Introduction
2. Materials and Methods
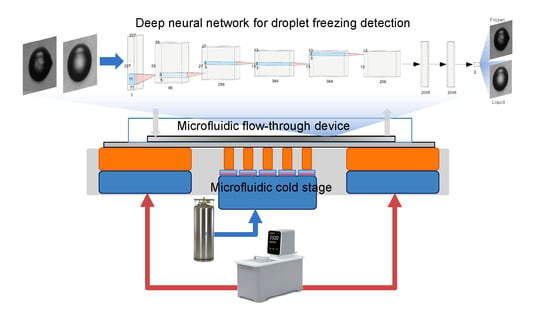
2.1. Temperature-Controlled Platform
2.2. Platinum Thin Film Temperature Sensor Design and Fabrication
2.3. Microfluidic Device Design and Fabrication
2.4. Chemicals Used
2.5. Aging, Heat Treatment and Fourier Transform Infrared Spectroscopy (FTIR) Study Methodology
3. Results
3.1. Polarized Intensity Threshold Algorithm for Freezing Detection
3.2. Deep Neural Network Algorithm for Freezing Detection
3.3. Case Study: Snomax® Ice Nucleation Detection

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, C.; Purnell, G.; James, S.J. A Review of Novel and Innovative Food Freezing Technologies. Food Bioprocess Technol. 2015, 8, 1616–1634. [Google Scholar] [CrossRef]
- Kiani, H.; Sun, D.-W. Water crystallization and its importance to freezing of foods: A review. Trends Food Sci. Technol. 2011, 22, 407–426. [Google Scholar] [CrossRef]
- Geidobler, R.; Winter, G. Controlled ice nucleation in the field of freeze-drying: Fundamentals and technology review. Eur. J. Pharm. Biopharm. 2013, 85, 214–222. [Google Scholar] [CrossRef]
- Karlsson, J.O.; Toner, M. Long-term storage of tissues by cryopreservation: Critical issues. Biomaterials 1996, 17, 243–256. [Google Scholar] [CrossRef]
- Manuchehrabadi, N.; Shi, M.; Roy, P.; Han, Z.; Qiu, J.; Xu, F.; Lu, T.J.; Bischof, J. Ultrarapid Inductive Rewarming of Vitrified Biomaterials with Thin Metal Forms. Ann. Biomed. Eng. 2018, 46, 1857–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa-Marzal, R.M.; Scherer, G.W. Advances in Understanding Damage by Salt Crystallization. Acc. Chem. Res. 2010, 43, 897–905. [Google Scholar] [CrossRef]
- McPherson, A. Protein Crystallization. In Protein Crystallography; Humana Press Inc.: New York, NY, USA, 2017; Volume 1607, pp. 17–50. ISBN 978-1-4939-7000-1. [Google Scholar]
- Berry, I.M.; Dym, O.; Esnouf, R.M.; Harlos, K.; Meged, R.; Perrakis, A.; Sussman, J.L.; Walter, T.S.; Wilson, J.; Messerschmidt, A. SPINE high-throughput crystallization, crystal imaging and recognition techniques: Current state, performance analysis, new technologies and future aspects. Acta Crystallogr. Sect. D Biol. Crystallogr. 2006, 62, 1137–1149. [Google Scholar] [CrossRef] [Green Version]
- Twomey, S. Pollution and the Planetary Albedo. Atmos. Environ. 2007, 41, 120–125. [Google Scholar] [CrossRef]
- Lohmann, U.; Feichter, J. Global indirect aerosol effects: A review. Atmos. Chem. Phys. 2005, 5, 715–737. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, B.A. Aerosols, Cloud Microphysics, and Fractional Cloudiness. Science 1989, 245, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.B. Cloud Microphysics and Climate. Science 1997, 276, 1072–1078. [Google Scholar] [CrossRef]
- Baker, M.B.; Peter, T. Small-scale cloud processes and climate. Nat. Cell Biol. 2008, 451, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; Wiley: Hoboken, NJ, USA, 2016; ISBN 978-1-118-94740-1. [Google Scholar]
- Pruppacher, H.R.; Klett, J.D. Microphysics of Clouds and Precipitation; Springer: Dordrecht, The Netherlands, 1997; ISBN 978-0-306-48100-0. [Google Scholar]
- Hartmann, D.L.; Ockert-Bell, M.E.; Michelsen, M.L. The Effect of Cloud Type on Earth’s Energy Balance: Global Analysis. J. Clim. 1992, 5, 1281–1304. [Google Scholar] [CrossRef] [Green Version]
- Pruppacher, H.R. A New Look at Homogeneous Ice Nucleation in Supercooled Water Drops. J. Atmos. Sci. 1995, 52, 1924–1933. [Google Scholar] [CrossRef] [Green Version]
- Kanji, Z.A.; Ladino, L.A.; Wex, H.; Boose, Y.; Burkert-Kohn, M.; Cziczo, D.J.; Krämer, M. Overview of Ice Nucleating Particles. Meteorol. Monogr. 2017, 58, 1.1–1.33. [Google Scholar] [CrossRef] [Green Version]
- Knopf, D.A.; Alpert, P.A.; Wang, B. The Role of Organic Aerosol in Atmospheric Ice Nucleation: A Review. ACS Earth Space Chem. 2018, 2, 168–202. [Google Scholar] [CrossRef]
- DeMott, P.J.; Prenni, A.J.; Liu, X.; Kreidenweis, S.M.; Petters, M.D.; Twohy, C.H.; Richardson, M.S.; Eidhammer, T.; Rogers, D.C. Predicting global atmospheric ice nuclei distributions and their impacts on climate. Proc. Natl. Acad. Sci. USA 2010, 107, 11217–11222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, B.J.; Broadley, S.L.; Wilson, T.W.; Atkinson, J.D.; Wills, R.H. Heterogeneous freezing of water droplets containing kaolinite particles. Atmos. Chem. Phys. 2011, 11, 4191–4207. [Google Scholar] [CrossRef] [Green Version]
- Hill, T.C.J.; Moffett, B.F.; DeMott, P.J.; Georgakopoulos, D.G.; Stump, W.L.; Franc, G.D. Measurement of Ice Nucleation-Active Bacteria on Plants and in Precipitation by Quantitative PCR. Appl. Environ. Microbiol. 2013, 80, 1256–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, T.C.J.; DeMott, P.J.; Tobo, Y.; Fröhlich-Nowoisky, J.; Moffett, B.F.; Franc, G.D.; Kreidenweis, S.M. Sources of organic ice nucleating particles in soils. Atmos. Chem. Phys. 2016, 16, 7195–7211. [Google Scholar] [CrossRef] [Green Version]
- DeMott, P.J.; Hill, T.C.J.; Petters, M.D.; Bertram, A.K.; Tobo, Y.; Mason, R.H.; Suski, K.J.; McCluskey, C.S.; Levin, E.J.T.; Schill, G.P.; et al. Comparative measurements of ambient atmospheric concentrations of ice nucleating particles using multiple immersion freezing methods and a continuous flow diffusion chamber. Atmos. Chem. Phys. 2017, 17, 11227–11245. [Google Scholar] [CrossRef] [Green Version]
- Kanji, Z.A.; Abbatt, J.P.D. The University of Toronto Continuous Flow Diffusion Chamber (UT-CFDC): A Simple Design for Ice Nucleation Studies. Aerosol Sci. Technol. 2009, 43, 730–738. [Google Scholar] [CrossRef]
- Hartmann, S.; Niedermeier, D.; Voigtländer, J.; Clauss, T.; Shaw, R.A.; Wex, H.; Kiselev, A.; Stratmann, F. Homogeneous and heterogeneous ice nucleation at LACIS: Operating principle and theoretical studies. Atmos. Chem. Phys. 2011, 11, 1753–1767. [Google Scholar] [CrossRef] [Green Version]
- Möhler, O.; Benz, S.; Saathoff, H.; Schnaiter, M.; Wagner, R.; Schneider, J.; Walter, S.; Ebert, V.; Wagner, S. The effect of organic coating on the heterogeneous ice nucleation efficiency of mineral dust aerosols. Environ. Res. Lett. 2008, 3, 025007. [Google Scholar] [CrossRef]
- Zobrist, B.; Marcolli, C.; Pedernera, D.A.; Koop, T. Do atmospheric aerosols form glasses? Atmos. Chem. Phys. 2008, 8, 5221–5244. [Google Scholar] [CrossRef] [Green Version]
- Mael, L.E.; Busse, H.; Grassian, V.H. Measurements of Immersion Freezing and Heterogeneous Chemistry of Atmospherically Relevant Single Particles with Micro-Raman Spectroscopy. Anal. Chem. 2019, 91, 11138–11145. [Google Scholar] [CrossRef] [PubMed]
- Baustian, K.J.; Wise, M.E.; Tolbert, M.A. Depositional ice nucleation on solid ammonium sulfate and glutaric acid particles. Atmos. Chem. Phys. 2010, 10, 2307–2317. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, N.; Duft, D.; Kiselev, A.; Leisner, T. Contact freezing efficiency of mineral dust aerosols studied in an electrodynamic balance: Quantitative size and temperature dependence for illite particles. Faraday Discuss. 2013, 165, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Taji, K.; Tachikawa, M.; Nagashima, K. Laser trapping of ice crystals. Appl. Phys. Lett. 2006, 88, 141111. [Google Scholar] [CrossRef]
- Beall, C.M.; Stokes, M.D.; Hill, T.C.; DeMott, P.J.; Dewald, J.T.; Prather, K.A. Automation and heat transfer characterization of immersion mode spectroscopy for analysis of ice nucleating particles. Atmos. Meas. Tech. 2017, 10, 2613–2626. [Google Scholar] [CrossRef] [Green Version]
- Kunert, A.T.; Lamneck, M.; Helleis, F.; Pöschl, U.; Pöhlker, M.L.; Fröhlich-Nowoisky, J. Twin-plate Ice Nucleation Assay (TINA) with infrared detection for high-throughput droplet freezing experiments with biological ice nuclei in laboratory and field samples. Atmos. Meas. Tech. 2018, 11, 6327–6337. [Google Scholar] [CrossRef] [Green Version]
- Peckhaus, A.; Kiselev, A.; Hiron, T.; Ebert, M.; Leisner, T. A comparative study of K-rich and Na/Ca-rich feldspar ice-nucleating particles in a nanoliter droplet freezing assay. Atmos. Chem. Phys. 2016, 16, 11477–11496. [Google Scholar] [CrossRef] [Green Version]
- Budke, C.; Koop, T. BINARY: An optical freezing array for assessing temperature and time dependence of heterogeneous ice nucleation. Atmos. Meas. Tech. 2015, 8, 689–703. [Google Scholar] [CrossRef] [Green Version]
- Cook, F.; Lord, R.; Sitbon, G.; Stephens, A.; Rust, A.; Schwarzacher, W. A pyroelectric thermal sensor for automated ice nucleation detection. Atmos. Meas. Tech. 2020, 13, 2785–2795. [Google Scholar] [CrossRef]
- Metcalf, A.R.; Narayan, S.; Dutcher, C.S. A review of microfluidic concepts and applications for atmospheric aerosol science. Aerosol Sci. Technol. 2018, 52, 310–329. [Google Scholar] [CrossRef]
- Nandy, L.; Dutcher, C.S. Phase Behavior of Ammonium Sulfate with Organic Acid Solutions in Aqueous Aerosol Mimics Using Microfluidic Traps. J. Phys. Chem. B 2017, 122, 3480–3490. [Google Scholar] [CrossRef]
- Nandy, L.; Liu, S.; Gunsbury, C.; Wang, X.; Pendergraft, M.A.; Prather, K.A.; Dutcher, C.S. Multistep Phase Transitions in Sea Surface Microlayer Droplets and Aerosol Mimics using Microfluidic Wells. ACS Earth Space Chem. 2019, 3, 1260–1267. [Google Scholar] [CrossRef]
- Roy, P.; Mael, L.E.; Makhnenko, I.; Martz, R.; Grassian, V.H.; Dutcher, C.S. Temperature-Dependent Phase Transitions of Aqueous Aerosol Droplet Systems in Microfluidic Traps. ACS Earth Space Chem. 2020, 4, 1527–1539. [Google Scholar] [CrossRef]
- Tarn, M.D.; Sikora, S.N.F.; Porter, G.C.E.; O’Sullivan, D.; Adams, M.; Whale, T.F.; Harrison, A.D.; Vergara-Temprado, J.; Wilson, T.W.; Shim, J.-U.; et al. The study of atmospheric ice-nucleating particles via microfluidically generated droplets. Microfluid. Nanofluid. 2018, 22, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarn, M.D.; Sikora, S.N.F.; Porter, G.C.E.; Wyld, B.V.; Alayof, M.; Reicher, N.; Harrison, A.D.; Rudich, Y.; Shim, J.-U.; Murray, B.J. On-chip analysis of atmospheric ice-nucleating particles in continuous flow. Lab Chip 2020, 20, 2889–2910. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.C.E.; Sikora, S.N.F.; Shim, J.-U.; Murray, B.J.; Tarn, M.D. On-chip density-based sorting of supercooled droplets and frozen droplets in continuous flow. Lab Chip 2020, 20, 3876–3887. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, T.; Polen, M.; Cheng, P.; Ekambaram, V.; Somers, J.; Anna, S.L.; Sullivan, R.C. Development and characterization of a “store and create” microfluidic device to determine the heterogeneous freezing properties of ice nucleating particles. Aerosol Sci. Technol. 2019, 54, 79–93. [Google Scholar] [CrossRef]
- Weng, L.; Tessier, S.N.; Smith, K.; Edd, J.F.; Stott, S.L.; Toner, M. Bacterial Ice Nucleation in Monodisperse D2O and H2O-in-Oil Emulsions. Langmuir 2016, 32, 9229–9236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häusler, T.; Witek, L.; Felgitsch, L.; Hitzenberger, R.; Grothe, H. Freezing on a Chip—A New Approach to Determine Heterogeneous Ice Nucleation of Micrometer-Sized Water Droplets. Atmosphere 2018, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Stan, C.A.; Schneider, G.F.; Shevkoplyas, S.S.; Hashimoto, M.; Ibanescu, M.; Wiley, B.J.; Whitesides, G.M. A microfluidic apparatus for the study of ice nucleation in supercooled water drops. Lab Chip 2009, 9, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Liu, S.; Dutcher, C.S. Droplet Interfacial Tensions and Phase Transitions Measured in Microfluidic Channels. Annu. Rev. Phys. Chem. 2021, 72, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Polen, M.; Lawlis, E.; Sullivan, R.C. The unstable ice nucleation properties of Snomax® bacterial particles. J. Geophys. Res. Atmos. 2016, 121, 11–666. [Google Scholar] [CrossRef]
- Shao, Q.; O’Flanagan, S.; Lam, T.; Roy, P.; Pelaez, F.; Burbach, B.J.; Azarin, S.M.; Shimizu, Y.; Bischof, J.C. Engineering T cell response to cancer antigens by choice of focal therapeutic conditions. Int. J. Hyperth. 2019, 36, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Roy, P.; Shao, Q.; Jiang, C.; Choi, J.; Chung, C.; Mehra, D.; Bischof, J.C. The Role of Protein Loss and Denaturation in Determining Outcomes of Heating, Cryotherapy, and Irreversible Electroporation on Cardiomyocytes. J. Biomech. Eng. 2018, 140. [Google Scholar] [CrossRef]
- Pelaez, F.; Manuchehrabadi, N.; Roy, P.; Natesan, H.; Wang, Y.; Racila, E.; Fong, H.; Zeng, K.; Silbaugh, A.M.; Bischof, J.C.; et al. Biomaterial scaffolds for non-invasive focal hyperthermia as a potential tool to ablate metastatic cancer cells. Biomaterials 2018, 166, 27–37. [Google Scholar] [CrossRef]
- Stan, C.A.; Tang, S.K.Y.; Whitesides, G.M. Independent Control of Drop Size and Velocity in Microfluidic Flow-Focusing Generators Using Variable Temperature and Flow Rate. Anal. Chem. 2009, 81, 2399–2402. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S. Gas Assisted Thin-Film Evaporation from Confined Spaces. Ph.D. Dissertation, Georgia Institute of Technology, Atlanta, GA, USA, 2011. [Google Scholar]
- Wood, S.E.; Baker, M.B.; Swanson, B.D. Instrument for studies of homogeneous and heterogeneous ice nucleation in free-falling supercooled water droplets. Rev. Sci. Instrum. 2002, 73, 3988–3996. [Google Scholar] [CrossRef]
- Ray, M.; Nesnidal, M.; Socha, D. Optical Detection of Airborne Ice Crystals and Liquid Water Droplets. In Proceedings of the 1st AIAA Atmospheric and Space Environments Conference, San Antonio, TX, USA, 22–25 June 2009. [Google Scholar] [CrossRef]
- Prileszky, T.A.; Furst, E.M. Crystallization Kinetics of Partially Crystalline Emulsion Droplets in a Microfluidic Device. Langmuir 2016, 32, 5141–5146. [Google Scholar] [CrossRef] [PubMed]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet classification with deep convolutional neural networks. Commun. ACM 2017, 60, 84–90. [Google Scholar] [CrossRef]
- Tobo, Y. An improved approach for measuring immersion freezing in large droplets over a wide temperature range. Sci. Rep. 2016, 6, 32930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wex, H.; Augustin-Bauditz, S.; Boose, Y.; Budke, C.; Curtius, J.; Diehl, K.; Dreyer, A.; Frank, F.; Hartmann, S.; Hiranuma, N.; et al. Intercomparing different devices for the investigation of ice nucleating particles using Snomax® as test substance. Atmos. Chem. Phys. 2015, 15, 1463–1485. [Google Scholar] [CrossRef] [Green Version]
- Beydoun, H.; Polen, M.; Sullivan, R.C. A new multi-component heterogeneous ice nucleation model and its application to Snomax bacterial particles and a Snomax-illte mineral particle mixture. Atmos. Chem. Phys. 2017, 17, 13545–13557. [Google Scholar] [CrossRef] [Green Version]
- Vali, G. Quantitative Evaluation of Experimental Results an the Heterogeneous Freezing Nucleation of Supercooled Liquids. J. Atmos. Sci. 1971, 28, 402–409. [Google Scholar] [CrossRef]
- Turner, M.A.; Arellano, F.; Kozloff, L.M. Three separate classes of bacterial ice nucleation structures. J. Bacteriol. 1990, 172, 2521–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwidetzky, R.; Kunert, A.T.; Bonn, M.; Pöschl, U.; Ramlov, H.; Devries, A.L.; Fröhlich-Nowoisky, J.; Meister, K. Inhibition of Bacterial Ice Nucleators Is Not an Intrinsic Property of Antifreeze Proteins. J. Phys. Chem. B 2020, 124, 4889–4895. [Google Scholar] [CrossRef]
- Hindmarsh, J.; Russell, A.; Chen, X. Experimental and numerical analysis of the temperature transition of a suspended freezing water droplet. Int. J. Heat Mass Transf. 2003, 46, 1199–1213. [Google Scholar] [CrossRef]
- Graeber, G.; Schutzius, T.M.; Eghlidi, H.; Poulikakos, D. Spontaneous self-dislodging of freezing water droplets and the role of wettability. Proc. Natl. Acad. Sci. USA 2017, 114, 11040–11045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, A.; Enríquez, O.R.; Brunet, P.; Colinet, P.; Snoeijer, J.H. Universality of Tip Singularity Formation in Freezing Water Drops. Phys. Rev. Lett. 2014, 113, 054301. [Google Scholar] [CrossRef] [Green Version]
- Warren, G.; Corotto, L. The consensus sequence of ice nucleation proteins from Erwinia herbicola, Pseudomonas fluorescens and Pseudomonas syringae. Gene 1989, 85, 239–242. [Google Scholar] [CrossRef]
- Davies, P.L. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem. Sci. 2014, 39, 548–555. [Google Scholar] [CrossRef]
- Garnham, C.P.; Campbell, R.L.; Walker, V.K.; Davies, P.L. Novel dimeric β-helical model of an ice nucleation protein with bridged active sites. BMC Struct. Biol. 2011, 11, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Yan, Q.; Chen, J.; He, Y.; Wang, J.; Zhang, H.; Yu, Z.; Li, L. Molecular Characterization of an Ice Nucleation Protein Variant (InaQ) from Pseudomonas syringae and the Analysis of Its Transmembrane Transport Activity in Escherichia coli. Int. J. Biol. Sci. 2012, 8, 1097–1108. [Google Scholar] [CrossRef] [Green Version]
- Roeters, S.; Golbek, T.; Bregnhøj, M.; Drace, T.; Alamdari, S.; Roseboom, W.; Kramer, G.; Šantl-Temkiv, T.; Finster, K.; Woutersen, S.; et al. The Ice Nucleating Protein InaZ is Activated by Low Temperature. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lukas, M.; Schwidetzky, R.; Kunert, A.T.; Backus, E.H.; Pöschl, U.; Fröhlich-Nowoisky, J.; Bonn, M.; Meister, K. Interfacial Water Ordering Is Insufficient to Explain Ice-Nucleating Protein Activity. J. Phys. Chem. Lett. 2021, 12, 218–223. [Google Scholar] [CrossRef]
- Efimova, Y.M.; Haemers, S.; Wierczinski, B.; Norde, W.; Van Well, A.A. Stability of globular proteins in H2O and D2O. Biopolym. 2007, 85, 264–273. [Google Scholar] [CrossRef]
- Roy, P.; House, M.L.; Dutcher, C.S. Data from: “A Microfluidic Device for Automated High Throughput Detection of Ice Nucleation of Snomax®”; UC San Diego Library Digital Collections; Center for Aerosol Impacts on Chemistry of the Environment (CAICE): San Diego, CA, USA, 2021. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, P.; House, M.L.; Dutcher, C.S. A Microfluidic Device for Automated High Throughput Detection of Ice Nucleation of Snomax®. Micromachines 2021, 12, 296. https://doi.org/10.3390/mi12030296
Roy P, House ML, Dutcher CS. A Microfluidic Device for Automated High Throughput Detection of Ice Nucleation of Snomax®. Micromachines. 2021; 12(3):296. https://doi.org/10.3390/mi12030296
Chicago/Turabian StyleRoy, Priyatanu, Margaret L. House, and Cari S. Dutcher. 2021. "A Microfluidic Device for Automated High Throughput Detection of Ice Nucleation of Snomax®" Micromachines 12, no. 3: 296. https://doi.org/10.3390/mi12030296
APA StyleRoy, P., House, M. L., & Dutcher, C. S. (2021). A Microfluidic Device for Automated High Throughput Detection of Ice Nucleation of Snomax®. Micromachines, 12(3), 296. https://doi.org/10.3390/mi12030296