Binary Pectin-Chitosan Composites for the Uptake of Lanthanum and Yttrium Species in Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Pectin-Chitosan Binary Composites
2.2.1. Pectin-Chitosan Polyelectrolyte Complexes in Water
2.2.2. Sonication Assisted Synthesis of Pectin-Chitosan Composites in DMSO
2.3. Characterization of Composite Materials
2.3.1. TGA
2.3.2. FTIR Spectroscopy
2.3.3. 13C solid State NMR Spectroscopy
2.3.4. Particle Size, Polydispersity Index (PDI), and Zeta Potential Measurements
2.3.5. Uptake of Y (III) and La (III) by Pectin and Chitosan Composites
3. Results and Discussion
3.1. TGA Results for the Pectin, Chitosan, and Pectin-Chitosan Composites
3.2. FTIR Spectral Results
3.3. 13C Solid-State NMR Spectral Results
3.4. Particle Size, PDI, and ζ-Potential of Samples
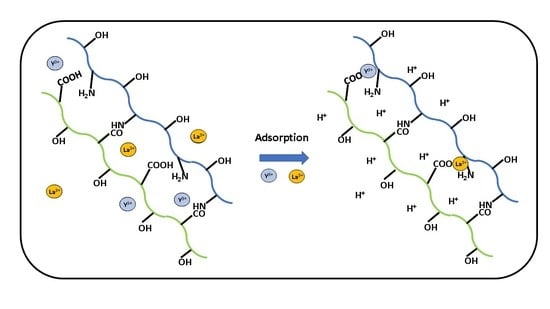
3.5. Sorption Isotherm Results
3.5.1. Uptake of Y (III) by Pectin-Chitosan Binary Composites
3.5.2. Uptake of La (III) by Pectin-Chitosan Binary Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Zhao, B.; Schreiner, B. Separation Hydrometallurgy of Rare Earth Elements; Springer International Publishing Switzerland: Cham, Switzerland, 2016. [Google Scholar]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Kusrini, E.; Usman, A.; Sani, F.A.; Wilson, L.D.; Abdullah, M.A.A. Simultaneous adsorption of lanthanum and yttrium from aqueous solution by durian rind biosorbent. Environ. Monit. Assess. 2019, 191, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths; CRC Press: New York, NY/Washington, DC, USA, 2004. [Google Scholar]
- Marczenco, Z. Separation and Spectrophotometric Determination of Elements; Ellis Horwood Ltd.: Chichester, UK, 1986; pp. 149–158. [Google Scholar]
- Chen, J.; Algeo, T.J.; Zhao, L.; Chen, Z.-Q.; Cao, L.; Zhang, L.; Li, Y. Diagenetic uptake of rare earth elements by bioapatite, with an example from Lower Triassic conodonts of South China. Earth Sci. Rev. 2015, 149, 181–202. [Google Scholar] [CrossRef]
- Ogata, T.; Narita, H.; Tanaka, M. Adsorption behavior of rare earth elements on silica gel modified with diglycol amic acid. Hydrometallurgy 2015, 152, 178–182. [Google Scholar] [CrossRef]
- Kusrini, E.; Sofyan, N.; Suwartha, N.; Yesya, G.; Priadi, C.R. Chitosan-praseodymium complex for adsorption of fluoride ions from water. J. Rare Earths 2015, 33, 1104–1113. [Google Scholar] [CrossRef]
- Kusrini, E.; Kinastiti, D.D.; Wilson, L.D.; Usman, A.; Rahman, A. Adsorption of Lanthanide Ions from an Aqueous Solution in Multicomponent Systems using Activated Carbon from Banana Peels. Int. J. Technol. 2018, 6, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Crini, G.; Torri, G.; Lichtfouse, E.; Kyzas, G.; Wilson, L.D.; Crini, N. Dye removal by biosorption using cross-linked chitosan-based hydrogels. Environ. Chem. Lett. 2019, 17, 1645–1666. [Google Scholar] [CrossRef] [Green Version]
- Wilson, L.D.; Tewari, B.B. Chitosan-based adsorbents: Environmental applications for the removal of arsenicals. In Chitosan-Based Adsorbents for Wastewater Treatment; Nasar, A., Ed.; Materials Research Forum: Aligarh, India, 2018; Volume 34, pp. 133–160. [Google Scholar]
- Hamza, M.F.; Gamal, A.; Hussein, G.; Nagar, M.S.; Abdel-Rahman, A.; Wei, Y.; Guibal, E. Uranium(VI) and zirconium(IV) sorption on magnetic chitosan derivatives-effect of different functional groups on separation properties. J. Chem. Tech. Biotech. 2019, 94, 3866–3882. [Google Scholar] [CrossRef]
- Kong, D.; Wilson, L.D. Uptake of Methylene Blue from Aqueous Solution by Pectin-Chitosan Binary Composites. J. Compos. Sci. 2020, 4, 95. [Google Scholar] [CrossRef]
- Tian, L.; Singh, A.; Singh, A.V. Synthesis and characterization of pectin-chitosan conjugate for biomedical application. Int. J. Biol. Macromol. 2020, 153, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Ding, G.; Geng, Q.; Zhu, J.; Guo, M.; Duan, Y.; Wang, B.; Cao, Y. Synthesis, Characterization, and Application of Microbe-Triggered Controlled-Release Kasugamycin–Pectin Conjugate. J. Agric. Food Chem. 2015, 63, 4263–4268. [Google Scholar] [CrossRef] [PubMed]
- Pasanphan, W.; Buettner, G.; Chirachanchai, S. Chitosan gallate as a novel potential polysaccharide antioxidant: An EPR study. Carbohydr. Res. 2010, 345, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marudova, M.; MacDougall, A.J.; Ring, S.G. Pectin-Chitosan interactions and gel formation. Carbohydr. Res. 2004, 339, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, H.; Zhu, X.; Hou, Y.; Liu, W.; Yang, G.; Jiang, A. Pectin-chitosan complex: Preparation and application in colon-specific capsule. Int. J. Agric. Biol. Eng. 2015, 8, 151–160. [Google Scholar]
- Chen, H.; Yang, W.; Chen, H.; Liu, L.; Gao, F.; Yang, X.; Jiang, Q.; Zhang, Q.; Wang, Y. Surface modification of Mitoxantrone-loaded PLGA nanospheres with chitosan. Colloids Surf. B 2009, 73, 212–218. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Zhao, Y. Biosorption and reduction of Au (III) to gold nanoparticles by thiourea modified alginate. Carbohydr. Polym. 2016, 159, 108–115. [Google Scholar] [CrossRef]
- Bradshaw, M.; Zou, J.; Byrne, L.; Iyer, K.S.; Stewart, S.G.; Raston, C.L. Pd(II) conjugated chitosan nanofibre mats for application in Heck cross-coupling reactions. Chem. Commun. 2011, 47, 12292–12294. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Yilmaz, O.; Yilmaz, E. Photoinduced Graft Copolymerization onto Chitosan under Heterogeneous Conditions. J. Macromol. Sci. A 2012, 49, 591–598. [Google Scholar] [CrossRef]
- Younes, I.; Hajji, S.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. Int. J. Biol. Macromol. 2014, 69, 489–498. [Google Scholar] [CrossRef]
- Lopes, I.S.; Michelon, M.; Duarte, L.G.R.; Prediger, P.; Cunha, R.L.; Picone, C.S.F. Effect of chitosan structure modification and complexation to whey protein isolate on oil/water interface stabilization. Chem. Eng. Sci. 2021, 230, 1–8. [Google Scholar] [CrossRef]
- Nurdjanah, S.; Hook, J.; Paton, J.; Paterson, J. Galacturonic Acid Content and Degree of Esterification of Pectin from Sweet Potato Starch Residue Detected Using 13C CP/MAS Solid State NMR. Eur. Food Res. Technol. 2013, 3, 16–37. [Google Scholar]
- Synytsya, A.; Copikova, J.; Brus, J. 13C CP/MAS NMR Spectra of Pectins: A Peak-Fitting Analysis in the C-6 Region. Czech J. Food Sci. 2003, 21, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Maciel, V.B.V.; Toshida, C.M.P.; Franco, T.T. Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr. Polym. 2015, 132, 537–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opanasopit, P.; Apirakaramwong, A.; Ruktanonchai, U. Development and Charaterization of Pectinate Micro/Nanoparticles for Gene Delivery. AAPS PharmSciTech 2008, 9, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Maciel, V.B.V.; Yoshida, C.M.P.; Pereira, S.M.S.S.; Goycoolea, F.M.; Franco, T.T. Electrostatic Self-Assembled Chitosan-Pectin Nano- and Microparticles for Insulin Delivery. Molecules 2017, 22, 1707. [Google Scholar] [CrossRef]
- Anisimov, Y.A.; Cree, D.E.; Wilson, L.D. Preparation of Multicomponent Biocomposites and Characterization of Their Physicochemical and Mechanical Properties. J. Compos. Sci. 2020, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Steiger, B.G.K.; Wilson, L.D. Modular Chitosan-Based Adsorbents for Tunable Uptake of Sulfate from Water. Int. J. Mol. Sci. 2020, 21, 7130. [Google Scholar] [CrossRef]
- Andishmand, H.; Tabibiazar, M.; Mohammadifar, M.A.; Hamishehkar, H. Pectin-zinc-chitosan-polyethylene glycol colloidal nano-suspension as a food grade carrier for colon targeted delivery of resveratrol. Int. J. Biol. Macromol. 2017, 97, 16–22. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, T.; Ding, S.; Wang, X. A novel cost-effective PAN/CNS nanofibrous membranes with rich carboxyl groups for high efficient adsorption of Lanthanum(III) ions. Sep. Purif. Technol. 2021, 259, 118216. [Google Scholar] [CrossRef]
- Li, P.; Xue, Y.; Liu, W.; Sun, G.; Ji, X. Chain structure comparison of two low density polyethylene resins fractionated by temperature rising elution fractionation and thermal fractionation. J. Polym. Res. 2019, 26, 1–13. [Google Scholar] [CrossRef]
- Guo, X.-G.; Yang, R.-O.; Gong, Y.; Jiang, Z.; Dong, Y.; Sun, X. Insights into the Coordination and Extraction of Yttrium(III) Ions with a Phenoxyacetic Acid Ionic-Liquid Extractant. Eur. J. Inorg. Chem. 2017, 2017, 2332–2339. [Google Scholar] [CrossRef]
- Qi, X.; Wang, Z.; Ma, S.; Wu, L.; Yang, S.; Xu, J. Complexation behavior of poly(acrylic acid) and lanthanide ions. Polymer 2014, 55, 1183–1189. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Fractionation of Carboxylate Anions from Aqueous Solution Using Chitosan Cross-Linked Sorbent Materials. RSC Adv. 2015, 5, 82065–82077. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, X.; Zhang, X.; Huang, L.; Liu, Z.; Sun, G. Highly efficient removal of trace metal ions by using poly(acrylic acid) hydrogel adsorbent. Mater. Des. 2019, 181, 107934. [Google Scholar] [CrossRef]







| Material | Temp. (°C) | Z-Avg. (d; nm) | PDI | ζ-Value (mV) | Conductivity (mS/cm) |
|---|---|---|---|---|---|
| Chitosan | 25 | 723.1 | 0.636 | 17.1 | 0.105 |
| PC 51 S | 25 | 992.0 | 0.286 | −31.4 | 0.216 |
| PC 51 W | 25 | 1808 | 0.785 | −6.37 | 0.0581 |
| PC 11 S | 25 | 1300 | 0.498 | −12.2 | 0.132 |
| PC 15 S | 25 | 1746 | 0.656 | −11.6 | 0.107 |
| Langmuir model best-fit parameters | |||
| KL | Qm | Adj. R-Square | |
| PC 15 S | 0.0063 ± 0.0029 | 20 ± 6.3 | 0.94 |
| PC 11 S | 0.013 ± 0.0022 | 19 ± 1.4 | 0.99 |
| PC 51 S | 0.033 ± 0.0060 | 23 ± 1.3 | 0.98 |
| Freundlich model best-fit parameters | |||
| KF | n | Adj. R-Square | |
| PC 15 S | 0.35 ± 0.16 | 1.4 ± 0.21 | 0.93 |
| PC 11 S | 0.83 ± 0.21 | 1.8 ± 0.19 | 0.96 |
| PC 51 S | 3.1 ± 0.59 | 2.6 ± 0.30 | 0.97 |
| Langmuir model best-fit parameters | |||
| KL | Qm | Adj. R-Square | |
| PC 15 S | 0.022 ± 0.0047 | 12 ± 0.77 | 0.97 |
| PC 11 S | 0.018 ± 0.0026 | 14 ± 0.70 | 0.98 |
| PC 51 S | 0.035 ± 0.0024 | 23 ± 0.40 | 0.99 |
| Freundlich model best-fit parameters | |||
| KF | n | Adj. R-Square | |
| PC 15 S | 1.4 ± 0.46 | 2.6 ± 0.47 | 0.94 |
| PC 11 S | 1.2 ± 0.30 | 2.3 ± 0.29 | 0.98 |
| PC 51 S | 4.2 ± 0.73 | 3.3 ± 0.40 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Kusrini, E.; Wilson, L.D. Binary Pectin-Chitosan Composites for the Uptake of Lanthanum and Yttrium Species in Aqueous Media . Micromachines 2021, 12, 478. https://doi.org/10.3390/mi12050478
Kong D, Kusrini E, Wilson LD. Binary Pectin-Chitosan Composites for the Uptake of Lanthanum and Yttrium Species in Aqueous Media . Micromachines. 2021; 12(5):478. https://doi.org/10.3390/mi12050478
Chicago/Turabian StyleKong, Dexu, Eny Kusrini, and Lee D. Wilson. 2021. "Binary Pectin-Chitosan Composites for the Uptake of Lanthanum and Yttrium Species in Aqueous Media " Micromachines 12, no. 5: 478. https://doi.org/10.3390/mi12050478
APA StyleKong, D., Kusrini, E., & Wilson, L. D. (2021). Binary Pectin-Chitosan Composites for the Uptake of Lanthanum and Yttrium Species in Aqueous Media . Micromachines, 12(5), 478. https://doi.org/10.3390/mi12050478